
A nomogram for predicting overall survival in patients with follicular thyroid cancer after thyroidectomy: a SEER database analysis
Introduction
Thyroid cancer (TC) is the most common malignant tumor of the neck, originating from thyroid follicular epithelial cells or parafollicular epithelial cells (1,2). In the past few decades, the incidence of TC has been increasing. The most common pathological type is papillary thyroid cancer (PTC), accounting for about 80% of cases, followed by follicular thyroid cancer (FTC), accounting for 10–15% of TC cases (3,4). Both PTC and FTC are types of differentiated thyroid carcinoma (DTC). They are often analyzed and treated as the type of TC. FTC is usually diagnosed by histopathological findings of vascular and/or capsule filling, but occasionally diagnosis is missed because of inadequate material or absence of a definite invasion (5,6). Due to the relatively low incidence of FTC and insufficient evidence from large samples and multicenter clinical trials, it is still unclear whether FTC and PTC should be treated equally (7).
FTC is a moderately differentiated carcinoma. Although the prognosis of patients after standardized treatment is good, 10% of patients still have recurrence and metastasis (7). Total thyroidectomy or subtotal thyroidectomy is the main treatment for FTC patients with or without distant metastasis (8). At present, the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system is the main criterion to guide the treatment and prognosis of FTC patients (9,10). However, traditional TNM staging is not effective in predicting the prognosis of individual patients (11). In addition, some other factors, including age, race, sex, treatment type, surgery, tumor size, and marital status, may also be important factors related to the prognosis of patients with FTC (12-14). Therefore, the combination of AJCC TNM staging and these variables may be better for predicting outcomes and would be better for clinical guidance.
Nomograms are a common tool to predict the prognosis of patients with cancers by incorporating and validating certain relevant factors (15). Previous studies have demonstrated that nomograms play an increasingly important role in predicting the outcomes of lung cancer, liver cancer, and other cancers (16-19). Moreover, in multiple cancers, nomograms that calculate the numerical probability of clinical events, such as cancer-specific survival (CSS) and overall survival (OS), have predicted more precisely than the traditional TNM staging systems. However, few studies have reported a nomogram model that can predict OS in patients with FTC after thyroidectomy.
In the present study, to predict prognosis for patients with FTC who underwent thyroidectomy, we developed and verified a nomogram based on several clinical variables using the Surveillance Epidemiology and End Results (SEER) database, which consists of research data from 20 regions in the United States (20). We present the following article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-386/rc).
Methods
Data source
The clinical data of 802 patients initially diagnosed with FTC was collected from the SEER database between 2010 and 2015. A nomogram to predict the prognosis of FTC patients at 3- and 5-year was established by the statistical analysis of regression model. Then, we measured the Harrell’s concordance index (C-index) to quantify the indication ability of the nomogram. Moreover, the survival rate between the actual survival rate and the predicted probability of survival by the nomogram was calibrated by a calibration curve. The inclusion criteria were as follows: (I) the diagnosis of FTC was made by histopathological examination; (II) patients with known survival months, cause of death, complete dates, and follow-up records; (III) sufficient information on variables including age, sex, race, grade, TNM staging, radiotherapy, chemotherapy, lymphadenectomy, tumor size, and marital status, among others. The exclusion criteria were as follows: (I) patients with benign tumors; (II) controversial cases; (III) patients with missing follow-up records or cause of death.
Ethical statement
This study was conducted on the basis of public data from the SEER database. Therefore, we have applied for the waive of the ethics committee agreement due to no personal identifying information being involved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Clinical characteristics
Some information was gained from the SEER dataset: patient demographics, tumor characteristics, therapeutic patterns, and survival results. The patient demographic analysis was used with age, sex, race, and marital status. Histological type, tumor stage, grade, as well as surgical information, were used in the analysis of tumor characteristics. We categorized the patients into several groups according to age (≤30, 31–40, 41–50, 51–60, 61–70, and >70 years). Sex was classified as female and male. We categorized the marital status as married and unmarried. Race was classified as black, white, and others (including American Indians, Asian, Alaskan natives, Pacific Islanders). Tumor size also was classified as 3 groups (cm): <2.0, 2.1–4.0, and ≥4.0. The TNM staging system was the AJCC 7th surgical-pathological staging classification. The World Health Organization histological classification and the ICD-0-3 site/histology validation list were used to identify FTC (8330/3). Tumor grade was divided into G1–G4, indicating respectively as undifferentiation,poor differentiation, moderate differentiation and well differentiation. In addition, radiotherapy, chemotherapy, and lymphadenectomy were all divided into treatment group and untreated group.
Statistical analysis
We analyzed the following variables for each patient: age, sex, race, grade, TNM stage, radiotherapy, chemotherapy, tumor size, and marital status. The Chi-square test was used to compare the patient baseline characteristics. Furthermore, the association between survival and different variables in patients with FTC was evaluated by univariate and multivariate Cox proportional hazards regression models with a hazard ratio (HR) and corresponding 95% confidence interval (CI). Modeling a nomogram with R software (version 3.4.5; https://www.r-project.org/) and all data were processed by SPSS 24.0 (IBM, USA). All statistical tests were two-sided, and P<0.05 indicated that the difference was statistically significant.
Results
Patient characteristics
A total of 802 FTC patients from the SEER database fulfilled the inclusion criteria and were enrolled in this study. Patients were randomly divided into two groups according to the proportion of 7:3 by R software. A total of 562 patients were included in the training set and 240 patients were included in the validation set. We reported the specific baseline clinicopathological variables in Table 1. Overall, the proportion of patients of different ages was equal. Most of the patients were female (71.4%) and white (76.9%). Moreover, G1 patients accounted for 77.3%. Additionally, 20.2% of patients were T1 stage, 39.7% of patients were T2 stage, 36.0% of patients were T3 stage, and 4.1% of patients were T4 stage. A total of 96.1% of patients had negative lymph nodes and 94.0% of patients had no distant metastasis. In addition, 66% of patients were treated with radiotherapy and only 0.7% of patients received chemotherapy. A total of 521 patients (65%) were treated with surgery without lymphadenectomy. No significant difference was observed in variables including age, sex, race, grade, T stage, N stage, M stage, radiotherapy, chemotherapy, tumor size, and marital status between the two groups (all P>0.05).
Table 1
| Variables | All patients (n=802) | Training set (n=562) | Validation set (n=240) | P value |
|---|---|---|---|---|
| Age (years), n (%) | 0.597 | |||
| ≤30 | 110 (13.7) | 84 (14.9) | 26 (10.8) | |
| 31–40 | 120 (15.0) | 84 (14.9) | 36 (15.0) | |
| 41–50 | 151 (18.8) | 105 (18.7) | 46 (19.2) | |
| 51–60 | 160 (20.0) | 111 (19.8) | 49 (20.4) | |
| 61–70 | 138 (17.2) | 98 (17.4) | 40 (16.7) | |
| >70 | 123 (15.3) | 80 (14.2) | 43 (17.9) | |
| Sex, n (%) | 0.553 | |||
| Female | 573 (71.4) | 405 (72.1) | 168 (70.0) | |
| Male | 229 (28.6) | 157 (27.9) | 72 (30.0) | |
| Race, n (%) | 0.225 | |||
| White | 617 (76.9) | 430 (76.5) | 187 (77.9) | |
| Black | 115 (14.3) | 77 (13.7) | 38 (15.8) | |
| Other | 70 (8.7) | 55 (9.8) | 15 (6.3) | |
| Grade, n (%) | 0.239 | |||
| G1 | 620 (77.3) | 443 (78.8) | 177 (73.8) | |
| G2 | 113 (14.1) | 70 (12.5) | 43 (17.9) | |
| G3 | 61 (7.6) | 43 (7.7) | 18 (7.5) | |
| G4 | 8 (1.0) | 6 (1.1) | 2 (0.8) | |
| T stage, n (%) | 0.954 | |||
| T1 | 162 (20.2) | 112 (19.9) | 50 (20.8) | |
| T2 | 318 (39.7) | 221 (39.3) | 97 (40.4) | |
| T3 | 289 (36.0) | 205 (36.5) | 84 (35.0) | |
| T4 | 33 (4.1) | 24 (4.3) | 9 (3.8) | |
| N stage, n (%) | 0.61 | |||
| N0 | 771 (96.1) | 539 (95.9) | 232 (96.7) | |
| N1 | 31 (3.9) | 23 (4.1) | 8 (3.3) | |
| M stage, n (%) | 0.156 | |||
| M0 | 754 (94.0) | 524 (93.2) | 230 (95.8) | |
| M1 | 48 (6.0) | 38 (6.8) | 10 (4.2) | |
| Radiotherapy, n (%) | 0.548 | |||
| No | 273 (34.0) | 195 (34.7) | 78 (32.5) | |
| Yes | 529 (66.0) | 367 (65.3) | 162 (67.5) | |
| Chemotherapy, n (%) | 0.675 | |||
| No | 796 (99.3) | 557 (99.1) | 239 (99.6) | |
| Yes | 6 (0.7) | 5 (0.9) | 1 (0.4) | |
| Lymphadenectomy, n (%) | 0.034 | |||
| No | 521 (65.0) | 352 (62.6) | 169 (70.4) | |
| Yes | 281 (35.0) | 210 (37.4) | 71 (29.6) | |
| Tumor size (cm), n (%) | 0.701 | |||
| <2.0 | 147 (18.3) | 99 (17.6) | 48 (20.0) | |
| 2.0–4.0 | 329 (41.0) | 231 (41.1) | 98 (40.8) | |
| ≥4.0 | 326 (40.6) | 232 (41.3) | 94 (39.2) | |
| Marital status, n (%) | 0.920 | |||
| Married | 459 (57.2) | 321 (57.1) | 138 (57.5) | |
| Unmarried | 343 (42.8) | 241 (42.9) | 102 (42.5) |
Prognostic factor analysis of survival based on clinical variables
In the training set, the results of univariate analysis revealed that age (P<0.001), other race (P=0.002), G3 (P<0.001), G4 (P<0.001), T4 stage (P<0.001), N1 stage (P=0.001), and M1 stage (P<0.001) were associated with survival in FTC patients. However, sex, radiotherapy, chemotherapy, lymphadenectomy, tumor size, and marital status were not related to survival (all P>0.05). The univariate analysis results are listed in Table 2.
Table 2
| Variables | HR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.073 (1.048–1.098) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 0.987 (0.488–1.999) | 0.972 |
| Race | ||
| White | Reference | |
| Black | 1.234 (0.469–3.346) | 0.671 |
| Other | 3.301 (1.527–7.139) | 0.002 |
| Grade | ||
| G1 | Reference | |
| G2 | 1.234 (0.417–3.649) | 0.704 |
| G3 | 9.062 (4.326–18.986) | <0.001 |
| G4 | 17.471 (5.110–59.729) | <0.001 |
| T stage | ||
| T1 | Reference | |
| T2 | 0.342 (0.096–1.212) | 0.096 |
| T3 | 1.473 (0.571–3.797) | 0.423 |
| T4 | 12.341 (4.618–32.982) | <0.001 |
| N stage | ||
| N0 | Reference | |
| N1 | 4.597 (1.899–11.128) | 0.001 |
| M stage | ||
| M0 | Reference | |
| M1 | 8.391 (4.198–16.775) | <0.001 |
| Radiotherapy | ||
| No | Reference | |
| Yes | 0.783 (0.406–1.510) | 0.465 |
| Chemotherapy | ||
| No | Reference | |
| Yes | 2.117 (0.284–15.769) | 0.464 |
| Lymphadenectomy | ||
| No | Reference | |
| Yes | 1.552 (0.815–2.958) | 0.181 |
| Tumor size (cm) | ||
| <2.0 | Reference | |
| 2.0–4.0 | 0.569 (0.212–1.530) | 0.264 |
| ≥4.0 | 1.368 (0.581–3.219) | 0.473 |
| Marital status | ||
| Married | Reference | |
| Unmarried | 1.879 (0.980–3.602) | 0.058 |
OS, overall survival; FTC, follicular thyroid cancer; HR, hazard ratio; CI, confidence interval.
In the multivariate analysis (Table 3), age (HR =1.057; 95% CI: 1.030–1.085; P<0.001), other race (HR =4.189; 95% CI: 1.781–9.854; P=0.001), G3 (HR =3.734; 95% CI: 1.571–8.874; P=0.003), G4 (HR =7.980; 95% CI: 1.579–40.327; P=0.012), and M1 stage (HR =2.582; 95% CI: 1.182–5.642; P=0.017) were associated with poorer OS rates for patients with FTC.
Table 3
| Variables | HR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.057 (1.030–1.085) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 1.906 (0.677–5.365) | 0.222 |
| Other | 4.189 (1.781–9.854) | 0.001 |
| Grade | ||
| G1 | Reference | |
| G2 | 0.759 (0.218–2.645) | 0.666 |
| G3 | 3.734 (1.571–8.874) | 0.003 |
| G4 | 7.980 (1.579–40.327) | 0.012 |
| T stage | ||
| T1 | Reference | |
| T2 | 0.440 (0.123–1.579) | 0.208 |
| T3 | 0.861 (0.307–2.417) | 0.776 |
| T4 | 2.474 (0.690–8.869) | 0.164 |
| N stage | ||
| N0 | Reference | |
| N1 | 1.832 (0.610–5.504) | 0.28 |
| M stage | ||
| M0 | Reference | |
| M1 | 2.582 (1.182–5.642) | 0.017 |
OS, overall survival; FTC, follicular thyroid cancer; HR, hazard ratio; CI, confidence interval.
Construction and validation of the nomogram
A nomogram was established based on clinical variables including age, grade, race, and M stage in the multivariate analysis. The 3- and 5-year OS prediction was estimated by a weighted total score calculated from each variable (Figure 1A). Additionally, the TNM staging system was further developed to predict 3- and 5-year OS (Figure 1B). The performance of the two predictive models was internally validated by discrimination and calibration methods. The calibration plots of the nomogram based on age, grade, race, and M stage revealed a good correlation between observed OS and nomogram prediction 3-, 5-year survival (Figure 2A,2B). Moreover, the calibration plots of the TNM staging system also revealed a good correlation between observed OS and nomogram prediction 3-, 5-year survival (Figure 2C,2D). Notably, the nomogram based on age, grade, race, and M stage showed better accuracy, with a C-index of 0.904 (95% CI: 0.883–0.925), compared with the traditional TNM staging system, which had a C-index of 0.775 (95% CI: 0.732–0.818) (Table 4).
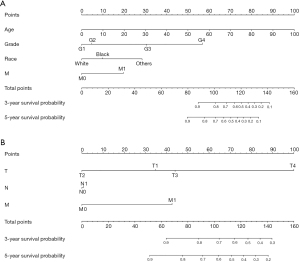
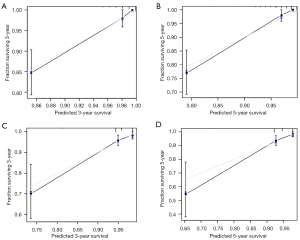
Table 4
| C-index compare | C-index (95% CI) | P value |
|---|---|---|
| Training set | ||
| Nomogram model | 0.904 (0.883–0.925) | <0.001 |
| TNM staging system | 0.775 (0.732–0.818) | <0.001 |
| Validation set | ||
| Nomogram model | 0.835 (0.772–0.898) | <0.001 |
| TNM staging system | 0.758 (0.685–0.832) | <0.001 |
C-index, concordance index; TNM, tumor-node-metastasis; CI, confidence interval.
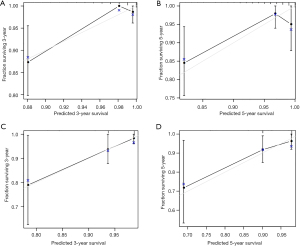
Next, we further validated the nomogram model in the validation set. The calibration plots of the nomogram based on age, grade, race, and M stage in the validation set also revealed a good correlation between observed OS and nomogram prediction 3-, 5-year survival (Figure 3A,3B). The calibration plots of the traditional TNM staging system showed a good correlation between observed OS and nomogram prediction 3-, 5-year survival (Figure 3C,3D). In the validation set, the nomogram based on age, grade, race, and M stage also revealed relatively better accuracy, with a C-index of 0.835 (95% CI: 0.772–0.898), compared with the TNM staging system, which had a C-index of 0.758 (95% CI: 0.685–0.832) (Table 4).
Discussion
The nomogram model constructed in this study incorporated several independent factors that may affect the prognosis of patients with FTC after thyroidectomy, including age, grade, race, and M stage. Compared with the traditional TNM staging system, the nomogram had better performance in terms of differentiation and calibration. The nomogram also had better accuracy in predicting the OS of patients with FTC after thyroidectomy.
In this study, age was a significant independent factor for survival in patients with FTC. Several studies have shown that the prognosis of elderly patients with TC is relatively poor (20-22). In addition, the relationship between older age and poor survival prognosis has been reported in many types of cancer (23-25). Highly DTC is the only malignant tumor that has been included in TNM staging by the AJCC (26). In a variety of staging systems, regardless of the cutoff, age is still identified as an important factor affecting the prognosis of patients with TC, which is consistent with our results.
Ethnic and racial disparities in health care exist and have been reported to be associated with worse prognosis in many diseases (27,28). A previous review has reported that black and white patients have a higher proportion of follicular cancer (14). The study by Asban et al. demonstrated significant gender and racial disparities in survival after TC surgery (13). In our study, we found that race other than white and black was a risk factor affecting the survival of FTC patients, which was consistent with the results of previous study (5).
Moreover, our results revealed that grade 3, grade 4, and M1 stage were independent risk factors for the prognosis of patients with FTC. In patients with FTC, distant metastasis is the strongest predictor of low OS. A previous study has reported that distant metastasis after surgery is an important factor affecting the death of patients with FTC (29). In addition, it has been demonstrated that distant metastasis at the initial diagnosis is an important factor affecting the poor survival of FTC patients, which is consistent with our results (30). Therefore, in clinical practice, clinicians should take active and effective treatment measures for patients with distant metastasis.
In the current study, we constructed a nomogram based on the above clinical variables that affected the survival of patients with FTC. Compared with the traditional TNM staging system, the C-index of the nomogram performed well in predicting the 3- and 5-year survival rates of FTC patients. According to the nomogram and risk score, clinicians can calculate an individual score for a patient and then predict the 3- and 5-year survival probability for FTC patients after thyroidectomy. To use the nomogram, we could draw a vertical line to mark the point row to allocate point values for each variable. Next, the total point is obtained by weighting the corresponding points. Finally, the total points correspond to the value of 3- and 5-year OS probability. More importantly, the nomogram is an easy-to-use (age, race, grade, and M stage) and inexpensive tool to assist clinicians to predict survival in patients with FTC after surgery in clinical practice.
However, there were some limitations that should be noted in this study. Firstly, this was a retrospective study, which may lead to missing data. Secondly, the overall mortality rate of patients with FTC was lower, so the assessment of the risk of recurrence was more meaningful than the risk of death. However, due to the limitation of the SEER database, the data related to postoperative recurrence could not be collected. Finally, due to the data originating from the SEER database, the included patients were mainly Westerners, and whether it would be applicable to Asian patients still needs to be verified by future research.
In summary, we have developed an intuitive and accurate tool to predict the prognosis of patients with FTC and their 3- and 5-year survival. This nomogram could provide corresponding treatment assistance for FTC patients and assist clinicians to make treatment decisions.
Acknowledgments
We would like to thank all those who have contributed to the research.
Funding: This study was funded by the Natural Science Foundation of Ningbo (Nos. 2021J313 and 202003N4250) and Thyroid Surgery-A Key Medical Discipline in Ningbo (No. 2022-F18).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-386/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-386/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet 2016;388:2783-95. [Crossref] [PubMed]
- Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid 2013;23:1015-21. [Crossref] [PubMed]
- Wiltshire JJ, Drake TM, Uttley L, et al. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid 2016;26:1541-52. [Crossref] [PubMed]
- Wang Z, Mo C, Chen L, et al. Application of competing risk model in the prognostic prediction study of patients with follicular thyroid carcinoma. Updates Surg 2022;74:735-46. [Crossref] [PubMed]
- Teo KW, Yuan NK, Tan WB, et al. Comparison of prognostic scoring systems in follicular thyroid cancer. Ann R Coll Surg Engl 2017;99:479-84. [Crossref] [PubMed]
- Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin 2013;63:374-94. [Crossref] [PubMed]
- Sugino K, Kameyama K, Ito K, et al. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid 2012;22:798-804. [Crossref] [PubMed]
- Lang BH, Lo CY, Chan WF, et al. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr Relat Cancer 2007;14:29-42. [Crossref] [PubMed]
- Lo CY, Chan WF, Lam KY, et al. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg 2005;242:708-15. [Crossref] [PubMed]
- Kim M, Kim HI, Jeon MJ, et al. Eighth edition of tumor-node-metastasis staging system improve survival predictability for papillary, but not follicular thyroid carcinoma: A multicenter cohort study. Oral Oncol 2018;87:97-103.
- Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988;104:947-53. [PubMed]
- Asban A, Chung SK, Xie R, et al. Gender and Racial Disparities in Survival After Surgery Among Papillary and Patients With Follicular Thyroid Cancer: A 45-Year Experience. Clin Med Insights Endocrinol Diabetes 2019;12:1179551419866196. [Crossref] [PubMed]
- Keane E, Francis EC, Catháin ÉÓ, et al. The role of race in thyroid cancer: systematic review. J Laryngol Otol 2017;131:480-6. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Wang S, Yang L, Ci B, et al. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J Thorac Oncol 2018;13:1338-48. [Crossref] [PubMed]
- Tian Y, He Y, Li X, et al. Novel nomograms to predict lymph node metastasis and distant metastasis in resected patients with early-stage non-small cell lung cancer. Ann Palliat Med 2021;10:2548-66. [Crossref] [PubMed]
- Chen Q, Mao R, Zhao J, et al. Nomograms incorporating preoperative RDW level for the prediction of postoperative complications and survival in colorectal liver metastases after resection. Ann Palliat Med 2021;10:4143-58. [Crossref] [PubMed]
- Wei FZ, Mei SW, Chen JN, et al. Nomograms and risk score models for predicting survival in rectal cancer patients with neoadjuvant therapy. World J Gastroenterol 2020;26:6638-57. [Crossref] [PubMed]
- Dasari A, Mehta K, Byers LA, et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018;124:807-15. [Crossref] [PubMed]
- Yan H, Winchester DJ, Prinz RA, et al. Differences in the Impact of Age on Mortality in Well-Differentiated Thyroid Cancer. Ann Surg Oncol 2018;25:3193-9. [Crossref] [PubMed]
- Saïe C, Wassermann J, Mathy E, et al. Impact of age on survival in radioiodine refractory differentiated thyroid cancer patients. Eur J Endocrinol 2021;184:667-76. [Crossref] [PubMed]
- Chon HS, Sehovic M, Marchion D, et al. Biologic Mechanisms Linked to Prognosis in Ovarian Cancer that May Be Affected by Aging. J Cancer 2019;10:2604-18. [Crossref] [PubMed]
- Zhang C, Liu L, Tao F, et al. Bone Metastases Pattern in Newly Diagnosed Metastatic Bladder Cancer: A Population-Based Study. J Cancer 2018;9:4706-11. [Crossref] [PubMed]
- Costa JG, de Jesus VHF, Camandaroba MPG, et al. Characteristics and survival of older patients with metastatic pancreatic cancer: a retrospective analysis of the AC Camargo Cancer Center experience. Ther Adv Med Oncol 2019;11:1758835919874650. [Crossref] [PubMed]
- Kong N, Xu Q, Zhang Z, et al. Age Influences the Prognosis of Anaplastic Thyroid Cancer Patients. Front Endocrinol (Lausanne) 2021;12:704596. [Crossref] [PubMed]
- Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health 2012;102:953-66. [Crossref] [PubMed]
- Sorkin DH, Ngo-Metzger Q, De Alba I. Racial/ethnic discrimination in health care: impact on perceived quality of care. J Gen Intern Med 2010;25:390-6. [Crossref] [PubMed]
- Patron V, Hitier M, Bedfert C, et al. Occult lymph node metastases increase locoregional recurrence in differentiated thyroid carcinoma. Ann Otol Rhinol Laryngol 2012;121:283-90. [Crossref] [PubMed]
- Passler C, Scheuba C, Prager G, et al. Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr Relat Cancer 2004;11:131-9. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


