
Robot-assisted and video-assisted thoracoscopic surgery for thymoma: comparison of the perioperative outcomes using inverse probability of treatment weighting method
Introduction
Mediastinal sternotomy has traditionally been the gold standard approach to mediastinal masses (1,2). However, minimally invasive surgery (MIS) has recently become the standard of treatment for early-stage thymoma. Several studies comparing perioperative outcomes between MIS and open surgery have reported the safety and feasibility of MIS (3-9). In addition, a few reports have stated that robot-assisted thoracoscopic surgery (RATS) results in a shorter postoperative hospital stay and duration of postoperative pleural drainage for early-stage thymoma than video-assisted thoracoscopic surgery (VATS) (10-13). However, no studies comparing RATS and VATS for thymoma have evaluated the postoperative quality of life (QOL). This study compared the improved postoperative QOL of RATS and VATS for thymoma based on assessment with nursing care systems using the inverse probability of treatment weighting (IPTW) method. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-333/rc).
Methods
Patients
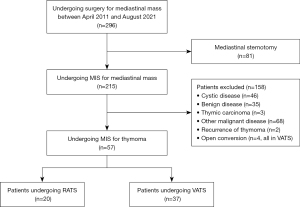
We retrospectively analyzed the medical records of 296 patients who underwent surgical resection of mediastinal masses in our hospital between April 2011 and August 2021. The patient flow chart with the inclusion and exclusion criteria is presented in Figure 1. We excluded patients with cystic disease, benign disease, other thymic neoplastic diseases, and/or recurrence. Although four open conversion cases were observed in VATS because of tumors larger than 8 cm and extensive tumor invasion into the lung, we excluded open conversion to avoid as much variability as possible between RATS and VATS. Ultimately, 57 patients who underwent MIS for thymoma (RATS: n=20; VATS: n=37) were included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Sapporo Medical University School of Medicine and Hospital (approval number: 332-112). The requirement of informed patient consent was waived because of the retrospective nature of the study.
Data collection
All patient data were collected via the medical records of our institution. The pathological stage of thymoma was evaluated based on the Masaoka classification and the eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer Tumor Node Metastasis (TNM) stage classification. Although T1 is usually composed of T1a and T1b, we collectively considered them as T1 in this study because T1 does not affect the surgical procedure regardless of the subclassification. Perioperatively, data on the following variables were collected: operation time, intraoperative bleeding, pleural drainage duration, postoperative pleural drainage, discontinuation of oxygen therapy, removal of a urinary balloon catheter, improved postoperative QOL, postoperative hospital stay, postoperative complications, mortality, and postoperative pain. Postoperative complications were graded using the Clavien-Dindo classification.
Evaluation of surgical outcomes
We evaluated improved postoperative QOL as the primary outcome. It was defined based on the duration until the patient could perform independent activities of daily living. We used two nursing care systems to evaluate this: the Nursing Dependency Score (NDS) and the Nursing Criteria (NC), as defined by the Ministry of Health, Labour and Welfare of Japan (Table 1) (14,15). The NDS measures the severity of the disease, activities of daily living, and the level of nursing care required by the patient. It is evaluated using the total score of three categories as follows: A. The treatment details for the patient; B. The level of independence and activity status; and C. Whether the patient is receiving acute care (14). Additionally, we also assessed the duration required to achieve a B item of fewer than 3 points (B duration). The NC evaluates patient independence during hospitalization and categorizes patients according to the need for nursing observation (A–C) and freedom of life (I–IV) (15). We assessed improved postoperative QOL by the duration required to achieve nursing criterion CIII (CIII duration).
Table 1
| Nursing care systems | Content |
|---|---|
| Nursing dependency score | |
| Turning over while sleeping in bed | |
| 0 point | Possible |
| 1 point | Possible if you hold on to something |
| 2 point | Impossible |
| Transferring | |
| 0 point | Possible |
| 1 point | Possible with some assistance |
| 2 point | Requiring full assistance |
| Intraoral cleaning | |
| 0 point | Possible |
| 1 point | Requiring assistance |
| 2 point | – |
| Food intake | |
| 0 point | Possible |
| 1 point | Possible with some assistance |
| 2 point | Requiring full assistance |
| Dressing and undressing | |
| 0 point | Possible |
| 1 point | Possible with some assistance |
| 2 point | Requiring full assistance |
| Understanding of medical instructions | |
| 0 point | Yes |
| 1 point | No |
| 2 point | – |
| Dangerous behavior | |
| 0 point | No |
| 1 point | – |
| 2 point | Yes |
| Nursing criteria | |
| Need for nursing observation | |
| A | Requires constant observation |
| B | Requires intermittent observation (about every 1–2 weeks) |
| C | No need for intermittent observation |
| Freedom of life | |
| I | Always lying in bed |
| II | Able to sit up in bed by themselves |
| III | Able to walk in the hospital |
| IV | Almost no inconvenience in daily life |
Improvement in postoperative QOL was presented as the durations required to achieve a B item of fewer than 3 points (B duration) and to achieve nursing criterion CIII (CIII duration), based on the Nursing Dependency Score and Nursing Criteria, respectively. QOL, quality of life.
For the secondary outcomes, we evaluated surgical outcomes including operation time, intraoperative bleeding, pleural drainage duration, postoperative pleural drainage (mL/kg/day), discontinuation of oxygen therapy, removal of a urinary balloon catheter, improved postoperative QOL, postoperative hospital stay, postoperative complications, and mortality. Particularly, mortality was defined as in-hospital 30-day or 90-day mortality.
Postoperative analgesia
We also evaluated postoperative pain and the prescription of analgesics during hospitalization and at the first and second outpatient follow-up visits. Epidural analgesia was generally used to control postoperative pain. At the discretion of the surgeon and anesthesiologist, the intercostal nerve block was performed in patients for whom epidural analgesia was avoided because of the use of antithrombotics or associated technically difficulty. In our institution, the first follow-up was conducted at 2–4 weeks postoperatively, and the second follow-up was conducted at 2–7 months after surgery. Postoperative pain grade in the outpatient department was defined as follows: (I) no pain or pain not requiring analgesics; (II) pain requiring analgesics (excluding those for neuralgia); (III) pain requiring analgesics for neuralgia [based on a modified version of a previously reported pain grade used in other thoracic fields (16)]. Non-steroidal anti-inflammatory drugs and acetaminophen were prescribed as regular analgesics during hospitalization and in the outpatient department. In addition, patients with neuralgia were treated with pregabalin or mirogabalin besylate, and patients with severe pain, regardless of regular analgesic use, were administered hydrochloride and fentanyl citrate tape until the pain was controlled.
Surgical technique
Between April 2011 and October 2018, a VATS approach with one window and two ports was performed for thymoma in all cases. The patient was placed in the left/right semi-decubitus position with both arms above the head. A mini-thoracotomy window was placed in the fifth intercostal space, and the ports were placed in the third and fourth intercostal spaces. Additional assistant ports were placed ipsilaterally or contralaterally according to the surgeon’s preference.
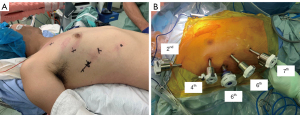
Thymic tissue dissection and thymic vein division were performed mostly using a vessel sealing system. From October 2018 to August 2021, the RATS approach was performed for thymoma using the da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) in all cases. The patient was placed in the left/right semi-decubitus position with the ipsilateral arm positioned backward (Figure 2A). Robotic 8-mm ports were placed between the second and eighth intercostal spaces according to the body size. Although three robotic arms were placed in the initial cases, four arms were placed from October 2019 onwards (Figure 2B). The first, second, third, and fourth arms were connected to the fenestrated bipolar forceps, 8-mm endoscope, Maryland bipolar forceps, and Vessel Sealer Extend, respectively (Intuitive Surgical Inc.). A port at the level of the sixth intercostal space was used as an assistant port with a 12-mm air seal port (Medical Leaders Co., Ltd., Tokyo, Japan). Intraoperative thoracic carbon dioxide (CO2) insufflation was set at a pressure of 8–10 mmHg. Thymothymectomy was performed by several different surgeons using the VATS approach and by two surgeons using the RATS approach. The thymus with the thymoma was removed from an assistant port or extended port incision of 30 mm or more due to the difficulty of removal from the 12-mm incision. A 19-Fr chest tube was generally inserted from the most caudal port without an additional utility incision. The chest tube was removed based on a postoperative pleural drainage volume of 5–6 mL/kg/day without air leakage. According to clinical pathway protocols, patients were discharged if they had no physical, mental, or social problems after undergoing chest X-rays and blood examination.
Statistical analysis
Continuous variables that did not follow a normal distribution are expressed as the median and interquartile range after performing the Shapiro-Wilk test for each variable to determine if it was normally distributed. In contrast, categorical variables are expressed as counts and percentages. All continuous variables were confirmed to have equal variances using the F-test. These variables were subsequently compared between the RATS and VATS groups using the Wilcoxon test and χ2 test. Furthermore, the IPTW method was used to account for residual differences in the selection bias of characteristics in the two groups. Propensity scores were calculated using 15 covariates of patient characteristics—age, sex, body height, body mass index, performance status, comorbidity of cardiovascular disease, diabetes mellitus, myasthenia gravis, use of antithrombotic, smoking, tumor size, operative procedure, combined resection, approach side, and adhesion. Patients in each group were weighted based on the propensity scores. To evaluate the efficacy of RATS compared to that of VATS, odds ratios (ORs) of postoperative outcomes with 95% confidence intervals (CI) were calculated using multivariable logistic regression analysis. Factors related to NDS and NC were also analyzed using multivariable logistic regression analysis. P values of 0.05 or less were considered statistically significant. Statistical analyses were performed using JMP Pro15 (SAS Institute, Cary, NC, USA).
Results
The baseline characteristics of the patients before and after IPTW are summarized in Table 2. Before and after IPTW adjustment, there were no significant differences between the two groups in age, sex, body height, body mass index, performance status, comorbidity of cardiovascular disease, diabetes mellitus, or myasthenia gravis, use of antithrombotics, smoking, or tumor size. All patients with myasthenia gravis had been confirmed positive for anti-acetylcholine receptor antibodies and had been treated preoperatively with prednisolone, pyridostigmine, and, in severe cases, intravenous immunoglobulins. All patients’ conditions were in a controlled state before undergoing surgery. Combined resections were required for two patients in the RATS group, involving a part of the lung; in the VATS group, this was required for four patients, involving a part of the lung in two patients, parts of the pericardium and the lung in one patient, and parts of the pericardium and the phrenic nerve in one patient. These distributions were not significantly different between the groups. There were no statistically significant differences in the operative procedure, the approach side, intraoperative adhesion, or adjuvant radiotherapy. However, RATS used more CO2 insufflation than VATS (P<0.001). All patients in both groups had sufficient oncological margins and were pathologically confirmed to have no tumor remnants (R0). Postoperative pathological outcomes based on the Masaoka classification, the TNM classification (T factor), and the World Health Organization classification were similar between the two groups (P=0.395, P=0.504, and P=0.284, respectively).
Table 2
| Characteristics | Before IPTW adjustment | After IPTW adjustment | |||||
|---|---|---|---|---|---|---|---|
| RATS (n=20) | VATS (n=37) | P value | RATS (n=20) | VATS (n=37) | P value | ||
| Age (years) | 55 (34 to 88) | 61 (30 to 82) | 0.646 | 55 (34 to 88) | 61 (30 to 82) | 0.651 | |
| Male/female (male) | 7/13 (35.0) | 16/21 (43.2) | 0.585 | 33.5 | 41.6 | 0.390 | |
| Body height (cm) | 159 (148 to 187) | 162 (149 to 186) | 0.900 | 159 (148 to 187) | 162 (149 to 186) | 0.907 | |
| Body mass index (kg/m2) | 24.1 (17.7 to 31.2) | 23.4 (16.0 to 32.7) | 0.676 | 24.1 (17.7 to 31.2) | 23.4 (16.0 to 32.7) | 0.682 | |
| Performance status 0–1 | 20 | 37 | – | 100 | 100 | – | |
| Comorbidities | |||||||
| Cardiovascular disease | 1 (5.0) | 2 (5.4) | 1.000 | 2.5 | 3.8 | 1.000 | |
| Diabetes mellitus | 3 (15.0) | 7 (18.9) | 1.000 | 21.2 | 20.9 | 0.804 | |
| Myasthenia gravis | 4 (20.0) | 11 (29.7) | 0.182 | 16.1 | 23.5 | 0.310 | |
| Use of antithrombotics | 2 (10.0) | 5 (13.5) | 1.000 | 4.9 | 9.5 | 0.695 | |
| Smoker | 0.830 | 0.687 | |||||
| Never | 9 (45.0) | 15 (40.5) | 45.6 | 43.3 | |||
| Former | 3 (15.0) | 8 (21.6) | 10.4 | 16.6 | |||
| Current | 8 (40.0) | 14 (37.8) | 44.1 | 40.1 | |||
| Tumor size (mm) | 33.5 (9 to 65) | 33 (9 to 70) | 0.442 | 33.5 (9 to 65) | 33 (9 to 70) | 0.447 | |
| Operative procedure | 0.286 | 0.132 | |||||
| Extended thymectomy | 6 (30.0) | 14 (37.8) | 33.3 | 35 | |||
| Thymectomy | 14 (70.0) | 20 (54.1) | 66.7 | 59.4 | |||
| Partial thymectomy | 0 | 3 (8.1) | 0 | 5.7 | |||
| Combined resection | 2 (10.0) | 4 (10.8) | 1.000 | 8.9 | 10.7 | 0.727 | |
| Approach side | 0.281 | 0.178 | |||||
| Right | 16 (80.0) | 28 (75.7) | 84.7 | 74.7 | |||
| Left | 4 (20.0) | 5 (13.5) | 15.3 | 17.7 | |||
| Bilateral | 0 | 4 (10.8) | 0 | 7.6 | |||
| Adhesion | 0.267 | 0.682 | |||||
| None | 13 (65.0) | 31 (83.8) | 78.8 | 85.5 | |||
| Mild | 5 (25.0) | 4 (10.8) | 13.4 | 8.5 | |||
| Moderate | 2 (10.0) | 2 (5.4) | 7.8 | 6 | |||
| Insufflation of carbon dioxide | 20 | 4 (10.8) | <0.001 | 100 | 14.2 | <0.001 | |
| Masaoka stages | 0.633 | 0.395 | |||||
| I | 10 (50.0) | 21 (56.8) | 52.5 | 54.2 | |||
| II | 10 (50.0) | 15 (40.5) | 47.5 | 41.7 | |||
| III/IV | 0/0 | 1 (2.7)/0 | 0/0 | 4.1/0 | |||
| TNM classification | |||||||
| T | 1.000 | 0.504 | |||||
| 1 | 20 (100.0) | 36 (97.3) | 100 | 95.9 | |||
| 2 | 0 | 1 (2.7) | 0 | 4.1 | |||
| N | 0 | 0 | – | 0 | 0 | – | |
| M | 0 | 0 | – | 0 | 0 | – | |
| Stage 1/2 | 20/0 | 36 (97.3)/1 (1.8) | 1.000 | 100.0/0 | 95.9/4.1 | 0.504 | |
| WHO | 0.923 | 0.284 | |||||
| A | 2 (10.0) | 4 (10.8) | 9.7 | 10 | |||
| AB | 7 (35.0) | 12 (32.4) | 30.5 | 28.9 | |||
| B1 | 5 (25.0) | 10 (27.0) | 22.4 | 35.7 | |||
| B2 | 5 (25.0) | 7 (18.9) | 33.8 | 16.5 | |||
| B3 | 1 (5.0) | 4 (10.8) | 3.6 | 8.9 | |||
| Adjuvant chemotherapy | 0 | 0 | – | 0 | 0 | – | |
| Adjuvant radiotherapy | 4 (20.0) | 7 (18.9) | 1.000 | 11.9 | 18.2 | 0.410 | |
Continuous data are presented as the median and interquartile range in brackets, and categorical data are presented as n (%) before IPTW adjustment, while as only % after IPTW adjustment. RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; IPTW, inverse probability of treatment weighting; TNM, tumor node metastasis; WHO, World Health Organization.
Details of port placement, incision, and the chest tube are shown in Table 3. Significantly more ports and intercostal incisions were used in the RATS group than in the VATS group (both P<0.001). Additionally, in the VATS group, a mini-thoracotomy window was placed more often, and the maximum incision length was longer than in the RATS group (both P<0.001). A bilateral VATS approach with one 35-mm mini-thoracotomy and two 12-mm ports was performed in four patients who required extended thymectomy at the surgeon’s discretion. There was no significant difference in the number of chest tube insertions between the two groups.
Table 3
| Variables | Before IPTW adjustment | After IPTW adjustment | |||||
|---|---|---|---|---|---|---|---|
| RATS (n=20) | VATS (n=37) | P value | RATS (n=20) | VATS (n=37) | P value | ||
| No. of ports | <0.001 | <0.001 | |||||
| 1–3 | 2 (10.0) | 33 (89.2) | 12.2 | 92.5 | |||
| 4–6 | 18 (90.0) | 4 (10.8) | 87.8 | 7.5 | |||
| Assistant port | 19 (95.0) | 26 (70.3) | 0.041 | 96.8 | 74.6 | 0.003 | |
| Mini-thoracotomy | 7 (35.0) | 35 (94.6) | <0.001 | 31.9 | 93.6 | <0.001 | |
| Max incision (mm) | 12 (8 to 40) | 35 (11 to 45) | <0.001 | 12 (8 to 40) | 35 (11 to 45) | <0.001 | |
| Max port incision (mm) | 12 (8 to 12) | 15 (11 to 20) | <0.001 | 12 (8 to 12) | 15 (11 to 20) | <0.001 | |
| No. of intercostal spaces | <0.001 | <0.001 | |||||
| <4 | 6 (30.0) | 34 (91.9) | 26.8 | 92.5 | |||
| 4–6 | 14 (70.0) | 3 (8.1) | 73.2 | 7.5 | |||
| No. of chest tubes | 1.000 | 0.504 | |||||
| 1 | 20 | 36 (97.3) | 100.0 | 95.9 | |||
| 2 | 0 | 1 (2.7) | 0 | 4.1 | |||
Continuous data are presented as the median and interquartile range in brackets, and categorical data are presented as n (%) before IPTW adjustment, while as only % after IPTW adjustment. IPTW, inverse probability of treatment weighting; RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
Surgical outcomes compared between the two groups before and after IPTW are summarized in Table 4 and their ORs are presented in Figure 3. After IPTW, the B duration and CIII duration were shorter in the RATS group than in the VATS group (P<0.001 and P=0.037, respectively). These superior results in the RATS group compared to the VATS group were confirmed on logistic regression analysis (OR 0.25, 95% CI: 0.10–0.63, P=0.003; and OR 0.31, 95% CI: 0.12–0.76, P=0.011, respectively).
Table 4
| Variables | Before IPTW adjustment | After IPTW adjustment | |||||
|---|---|---|---|---|---|---|---|
| RATS (n=20) | VATS (n=37) | P value | RATS (n=20) | VATS (n=37) | P value | ||
| Primary outcomes | |||||||
| B duration (days)a | 2 (1 to 4) | 3 (1 to 7) | <0.001 | 2 (1 to 4) | 3 (1 to 7) | <0.001 | |
| CIII duration (days)a | 3 (1 to 6) | 5 (1 to 14) | 0.018 | 3 (1 to 6) | 5 (1 to 14) | 0.037 | |
| Secondary outcomes | |||||||
| Operation time (minutes) | 176 (103 to 285) | 143 (68 to 385) | 0.024 | 176 (103 to 285) | 143 (68 to 385) | 0.025 | |
| Intraoperative bleeding (mL) | 10 (5 to 100) | 10 (5 to 390) | 0.328 | 10 (5 to 100) | 10 (5 to 390) | 0.332 | |
| Pleural drainage duration (days) | 1 (1 to 2) | 1 (1 to 6) | 0.065 | 1 (1 to 2) | 1 (1 to 6) | 0.067 | |
| Postoperative pleural drainage (mL/kg/day) | 3.3 (0.4 to 8.0) | 4.3 (1.0 to 9.9) | 0.707 | 3.3 (0.4 to 8.0) | 4.3 (1.0 to 9.9) | 0.713 | |
| Removal of oxygen (days) | 1 (1 to 8) | 1 (1 to 7) | 0.310 | 1 (1 to 8) | 1 (1 to 7) | 0.315 | |
| Removal of urinary balloon catheter (days) | 1 (1 to 2) | 1 (1 to 3) | 0.503 | 1 (1 to 2) | 1 (1 to 3) | 0.511 | |
| Postoperative hospital stay (days) | 8 (2 to 14) | 8 (2 to 18) | 0.407 | 8 (2 to 14) | 8 (2 to 18) | 0.412 | |
| Total complications | 4 (20.0) | 8 (21.6) | 1.000 | 20.7 | 18.5 | 0.599 | |
| Phrenic nerve palsy | 3 (15.0) | 3 (8.1) | 0.657 | 13.4 | 6.6 | 0.458 | |
| Intercostal neuralgia | 1 (5.0) | 2 (5.4) | 1.000 | 7.3 | 4.6 | 1.000 | |
| Pneumothorax | 0 | 1 (2.7) | 1.000 | 0 | 1.9 | 1.000 | |
| Atrial fibrillation | 0 | 1 (2.7) | 1.000 | 0 | 1.9 | 1.000 | |
| Disrupted wound healing | 0 | 1 (2.7) | 1.000 | 0 | 3.4 | 1.000 | |
| Myasthenic crisis | 0 | 0 | – | 0 | 0 | – | |
| Mortality (30-day) | 0 | 0 | – | 0 | 0 | – | |
| Mortality (90-day) | 0 | 0 | – | 0 | 0 | – | |
| Postoperative pain | |||||||
| During hospitalization | |||||||
| Epidural analgesia | 20 | 30 | 0.045 | 100 | 84.9 | 0.018 | |
| Removal of epidural anesthesia | 2 (1 to 4) | 2 (1 to 4) | 0.144 | 2 (1 to 4) | 2 (1 to 4) | 0.146 | |
| No. of injections of analgesics | 2 (0 to 4) | 2 (1 to 5) | 0.577 | 2 (0 to 4) | 2 (1 to 5) | 0.583 | |
| Additional regular prescription | 2 | 10 | 0.182 | 14.1 | 35.5 | 0.033 | |
| Prescription as needed | 4 | 9 | 1.000 | 10.6 | 28.4 | 0.035 | |
| In the first follow-up | |||||||
| Duration (days) | 25 (12 to 37) | 22 (12 to 97) | 0.085 | 25 (12 to 37) | 22 (12 to 97) | 0.087 | |
| Pain gradeb | 0.885 | 0.376 | |||||
| 1 | 10 (50.0) | 19 (51.4) | 38.9 | 45.5 | |||
| 2 | 9 (45.0) | 15 (40.5) | 52.4 | 39.1 | |||
| 3 | 1 (5.0) | 3 (8.1) | 8.7 | 15.5 | |||
| Prescription | 10 (52.6) | 17 (46.0) | 0.779 | 53.99 | 51.5 | 0.812 | |
| In the second follow-up | |||||||
| Duration (days) | 92 (22 to 214) | 116 (48 to 389) | 0.267 | 92 (22 to 214) | 116 (48 to 389) | 0.272 | |
| Pain gradeb | 0.700 | 0.109 | |||||
| 1 | 18 (90.0) | 32 (86.5) | 86.9 | 84.0 | |||
| 2 | 1 (5.0) | 4 (10.8) | 4.4 | 14.1 | |||
| 3 | 1 (5.0) | 1 (2.7) | 8.7 | 4.9 | |||
| Prescription | 2 (10.5) | 4 (10.8) | 1.000 | 13.8 | 10.4 | 1.000 | |
Continuous data are presented as the median and interquartile range in brackets, and categorical data are presented as n (%) before IPTW adjustment, while as only % after IPTW adjustment. a, improvement in postoperative QOL was presented as the durations required to achieve a B item of fewer than 3 points (B duration) and to achieve nursing criterion CIII (CIII duration), based on the Nursing Dependency Score and Nursing Criteria, respectively; b, pain grade was defined as follows: 1. no pain or pain not requiring analgesics; 2. pain requiring analgesics (excluding those for neuralgia); 3. pain requiring analgesics for neuralgia. RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; IPTW, inverse probability of treatment weighting; QOL, quality of life.
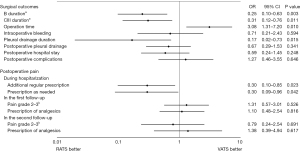
Although pleural drainage duration tended to be shorter in the RATS group than in the VATS group, the difference between the two groups after IPTW was not statistically significant (P=0.067). However, after adjustment for covariates on the logistic regression analysis, pleural drainage duration was shorter in the RATS group than in the VATS group (OR 0.17, 95% CI: 0.02–0.73, P=0.015). In addition, the RATS group had a longer operation time than the VATS group before and after IPTW (P=0.025). Moreover, after adjustment for covariates on the logistic regression analysis, there was a significant difference between the two groups (OR 3.08, 95% CI: 1.31–7.20, P=0.010). However, intraoperative bleeding, postoperative pleural drainage, discontinuation of oxygen therapy, removal of a urinary balloon catheter, or postoperative hospital stay were similar between the two groups.
The total complications in both groups were only of Clavien-Dindo grade 1, including four patients (20.7%) in the RATS group and eight patients (18.5%) in the VATS group. The difference between the two groups was not statistically significant after IPTW (P=0.599). In the VATS group, one patient developed postoperative pneumothorax requiring drainage, whereas no patients had this in the RATS group. Moreover, phrenic nerve paresis occurred in three patients (13.4%; two ipsilateral, one contralateral) who underwent RATS thymectomy without combined resection and three patients (6.6%; two ipsilateral, one contralateral) who underwent VATS thymectomy without combined resection. In the RATS group, one patient with contralateral phrenic nerve paresis improved approximately 1 year after surgery. No myasthenic crisis or mortality (30- and 90-day) occurred in either group.
Postoperative pain compared between the two groups before and after IPTW are summarized in Table 4 and their ORs are presented in Figure 3. Significantly fewer patients were prescribed epidural analgesia in the VATS group than in the RATS group (P=0.018) after IPTW. In contrast, additional analgesics (including those for wound pain and neuralgia) were prescribed significantly more often during postoperative hospitalization in the VATS group (P=0.033). There were no significant differences in the duration of epidural analgesia or the number of analgesic injections. The time intervals until the first and second follow-ups were similar in the two groups. The postoperative pain grade at the first and second follow-ups did not significantly differ between the two groups after IPTW (P=0.376 and P=0.109, respectively). Furthermore, there were no significant differences in the ORs of pain grades 2–3 at both follow-up periods (OR 1.31, 95% CI: 0.57–3.01, P=0.526 and OR 0.79, 95% CI: 0.24–2.54, P=0.691, respectively). Prescriptions of analgesics in both follow-up periods were also equal between the two groups (P=0.812 and P=1.000, respectively).
Multivariable logistic regression analysis of factors associated with NDS and NC for all patients after IPTW is summarized in Table 5. Pleural drainage duration was related to increased B and CIII durations (OR 73.48, 95% CI: 9.94–543.11, P<0.001 and OR 13.40, 95% CI: 2.36–76.92, P=0.004, respectively). There were no significant differences in extended thymectomy, bilateral approach, epidural analgesia, and prescription of analgesics (regular and as needed).
Table 5
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| B durationa | |||
| Operative procedure | |||
| Extended thymectomy | 10.07 | 0.77–131.25 | 0.078 |
| Thymectomy (including partial) | 1.00 | ||
| Approach side | |||
| Bilateral | 1.56 | 0.11–21.8 | 0.743 |
| Unilateral | 1.00 | ||
| Pleural drainage duration | 73.48 | 9.94–543.11 | <0.001 |
| Epidural analgesia | 0.81 | 0.01–55.5 | 0.923 |
| Additional regular prescription | 0.60 | 0.07–5.09 | 0.640 |
| Prescription as needed | 0.58 | 0.06–5.96 | 0.643 |
| CIII durationa | |||
| Operative procedure | |||
| Extended thymectomy | 1.53 | 0.56–4.16 | 0.408 |
| Thymectomy (including partial) | 1.00 | ||
| Approach side | |||
| Bilateral | 5.10 | 0.79–32.96 | 0.087 |
| Unilateral | 1.00 | ||
| Pleural drainage duration | 13.40 | 2.36–76.92 | 0.004 |
| Epidural analgesia | 0.70 | 0.13–3.80 | 0.678 |
| Additional regular prescription | 0.58 | 0.20–1.70 | 0.324 |
| Prescription as needed | 0.48 | 0.15–1.46 | 0.195 |
a, improvement in postoperative QOL was presented as the durations required to achieve a B item of fewer than 3 points (B duration) and to achieve nursing criterion CIII (CIII duration), based on the Nursing Dependency Score and Nursing Criteria, respectively. IPTW, inverse probability of treatment weighting; OR, odds ratio; CI, confidence interval; QOL, quality of life.
Discussion
In the 2017 annual report by the Japanese Association for Thoracic Surgery, the surgical case rate for thymomas was 37.3% (1,939/5,197) of mediastinal tumors, and the percentage of cases of thymoma treated with VATS was 63.0% (1,222/1,939) (17). Although conventional open surgery with median sternotomy has been used as the gold standard treatment for mediastinal masses, RATS and VATS have recently become alternative approaches for early-stage thymoma (3-5). Several studies have reported that RATS has advantages over open surgery in perioperative outcomes (6-9). Moreover, in the few studies comparing the two MIS approaches, RATS resulted in a shorter postoperative hospital stay and duration of postoperative pleural drainage for early-stage thymoma than VATS (10-12). However, no previous studies have compared and evaluated improved postoperative QOL between the MIS for thymoma, and therefore, we designed the present study.
In the present study, RATS offered the advantages of improved postoperative QOL based on the nursing care system, including NDS and NC. This result might be due to the longer pleural drainage duration in the VATS group than in the RATS group because one patient had postoperative pneumothorax in the VATS group. Furthermore, the subgroup analysis results indicated that prolonged pleural drainage duration might increase the nursing care durations. In addition, the difference in pain control during hospitalization might have affected NDS and NC; however, we could not evaluate this because of inadequate data on the visual analog scale scores during hospitalization. Compared to the RATS group, the use of epidural analgesia was significantly low and the rate of additional prescriptions was significantly high in the VATS group, suggesting that pain control with epidural analgesia contributed to the improved NDS and NC. Although epidural analgesia and analgesic prescriptions were not relevant to NDS and NC in the subgroup analysis, the present analysis could not sufficiently explain this relationship due to the small sample size.
In Japan, particularly in local hospitals, social factors often lead to longer postoperative hospital stays. Therefore, we consider that postoperative hospital stay is difficult to compare because of the variation in discharge criteria across institutions and the difference in medical systems across countries. In our study, RATS had shorter B duration and CIII duration than VATS based on the NDS and NC, respectively. Nevertheless, there was no difference in postoperative hospital stay between the groups. Consequently, postoperative QOL assessment using nursing care systems might be more useful and perceptive as an indicator to compare the different approaches. Most institutions in Japan use the NDS and NC to assess the QOL of patients during hospitalization. Several reports worldwide have used nursing systems as a scale to compare postoperative outcomes, even in intensive care units, such as the Therapeutic Intervention Scoring System, Nine Equivalents of Nursing Manpower Use Score, and Nursing Activities Score. Japanese nursing care systems are similar to these systems (18-20).
After IPTW, the OR obtained on logistic regression analysis in our study showed that the operation time was shorter in the VATS group than in the RATS group; however, several authors reported that the operation times of RATS and VATS were similar (10,12). One reason is the time specific to RATS, such as port insertion, targeting of the robotic camera by the assistant surgeon, and roll-in/out of the patient cart. For another reason, in RATS, changing the visualization angle in the mediastinum by switching the robotic port for insertion of the robotic camera from the second port to the third port facilitated dissection of the bilateral upper poles of the thymus and identification of the bilateral phrenic nerve compared to VATS. Therefore, RATS may have increased the resection area in the contralateral tissue and the bilateral upper poles of the thymus. Additionally, the VATS group included more patients undergoing partial thymectomy than the RATS group.
In a few published studies, the incidence of postoperative complications was comparable between RATS and VATS for thymoma, similar to our findings (15,16). In the present study, phrenic nerve paresis incidence was equal in both groups due to damage occurring while isolating the phrenic nerve as much as possible from invasion by the thymoma. There were no significant differences between the groups regarding postoperative myasthenic crisis because patients underwent proper preoperative treatment for myasthenia gravis. Furthermore, both approaches had no mortality and were safe.
Şehitogullari et al. reported that the postoperative pain and remission rates were similar between the RATS and VATS groups (11). However, no study on thymoma has reported data using pain grade. In the present study, the level of postoperative pain in the outpatient department was classified using pain grades based on analgesic use. We found no significant differences in pain between the groups at the first and second follow-ups, even though RATS used more ports and intercostal space access than VATS. Although these results might demonstrate that the number of ports and the number of intercostal spaces for port insertion were unrelated to postoperative pain, including wound pain and neuralgia, a further prospective study is needed to corroborate sufficient evidence in this regard.
Limitations
This study had some limitations. First, this was a single-center retrospective study, which resulted in selection bias, and the sample size was small, even though the F-test confirmed equal variances of each covariate. Furthermore, the present study included patients who had undergone surgery for thymoma for over 10 years, where the VATS approach was used in the first 8 years and the RATS approach was used in the latter 4 years. Although we used IPTW to decrease the selection bias, this limitation could not be completely addressed because clinical practices naturally changed over the decade. Second, as with all objective score systems, evaluations performed by nurses using the nursing care systems might not be consistent. Combining QOL assessment with subjective patient measures such as the European Organization for Research and Treatment of Cancer QOL Questionnaire could help overcome this limitation; however, this is not possible in a retrospective study (20). Finally, the same surgeon did not perform all procedures, and the procedure reflected the surgeon’s preference. Moreover, the prescription of analgesics was influenced by the preferences of the attending surgeon. To equalize these limitations as much as possible, we performed IPTW based on the propensity score of each covariate. IPTW is a statistical method in survival analysis used to homogenize patient characteristics and has been used in several previous studies (21-23). Although CO2 insufflation might be a confounder in our retrospective study, despite the use of IPTW, we could not eliminate it due to the loss of balanced propensity scores if this variable was included in the selection of covariates used to calculate this score.
Conclusions
The present study demonstrated RATS offered the advantages of improved postoperative QOL according to the nursing care systems compared to that with VATS. This study showed that the nursing care systems might be useful indicators of postoperative QOL assessment. Nevertheless, a further prospective study is warranted to present sufficient evidence for the study results.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-333/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-333/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-333/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Sapporo Medical University School of Medicine and Hospital (approval number: 332-112). The requirement of informed patient consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Yang CJ, Hurd J, Shah SA, et al. A national analysis of open versus minimally invasive thymectomy for stage I to III thymoma. J Thorac Cardiovasc Surg 2020;160:555-567.e15. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73. [Crossref] [PubMed]
- Buentzel J, Straube C, Heinz J, et al. Thymectomy via open surgery or robotic video assisted thoracic surgery: Can a recommendation already be made? Medicine (Baltimore) 2017;96:e7161. [Crossref] [PubMed]
- Casiraghi M, Galetta D, Borri A, et al. Robotic-assisted thymectomy for early-stage thymoma: a propensity-score matched analysis. J Robot Surg 2018;12:719-24. [Crossref] [PubMed]
- Marulli G, Comacchio GM, Schiavon M, et al. Comparing robotic and trans-sternal thymectomy for early-stage thymoma: a propensity score-matching study. Eur J Cardiothorac Surg 2018;54:579-84. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Şehitogullari A, Nasır A, Anbar R, et al. Comparison of perioperative outcomes of videothoracoscopy and robotic surgical techniques in thymoma. Asian J Surg 2020;43:244-50. [Crossref] [PubMed]
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Ministry of Health, Labour and Welfare. Nursing dependency score. Available online: https://www.mhlw.go.jp/content/12404000/000584563.pdf
- Uchida H, Sugaya Y, Gojo M. The development of a new objective and standardized system in nursing criteria. (in Japanese with English abstract). Kyotodaigaku Iryogijutsu Tankidaigakubu Kiyo 1992;12:3-13.
- Hsu HH, Liu YH, Chen HY, et al. Vicryl Mesh Coverage Reduced Recurrence After Bullectomy for Primary Spontaneous Pneumothorax. Ann Thorac Surg 2021;112:1609-15. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Padilha KG, Sousa RM, Kimura M, et al. Nursing workload in intensive care units: a study using the Therapeutic Intervention Scoring System-28 (TISS-28). Intensive Crit Care Nurs 2007;23:162-9. [Crossref] [PubMed]
- Robas Gómez A, Romero V, García García R, et al. Is the NEMS scale useful to describe homogeneously a population of patients in Intensive Care? Enferm Intensiva 2007;18:70-7. [PubMed]
- Padilha KG, de Sousa RM, Queijo AF, et al. Nursing Activities Score in the intensive care unit: analysis of the related factors. Intensive Crit Care Nurs 2008;24:197-204. [Crossref] [PubMed]
- Pompili C, Koller M, Velikova G, et al. EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2018;123:149-54. [Crossref] [PubMed]
- Sugihara M. Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharm Stat 2010;9:21-34. [Crossref] [PubMed]
- Tang EK, Chang JM, Chang CC, et al. Prognostic Factor of Completely Resected and Pathologic T3 N0 M0 Thymic Epithelial Tumor. Ann Thorac Surg 2021;111:1164-73. [Crossref] [PubMed]


