
Diagnostic performance of ultrasound and computed tomography in parallel for the diagnosis of lymph node metastasis in patients with thyroid cancer: a systematic review and meta-analysis
Introduction
The incidence of thyroid cancer (TC) has increased significantly in recent decades and has become a focus of research (1,2). Although there have been several advances in treatment over the past few decades (3), recurrence and metastasis are still unavoidable, especially in undifferentiated TC. Cervical lymph node metastasis (LNM), which occurs in regions of the neck, is a common metastasis of TC. It has a frequency of 30–90% in papillary TC (4). Early detection of LNM plays an important role in the clinical treatment plan (e.g., surgical method, surgical scope, postoperative adjuvant treatment, psychologic impact, and prognosis) (5-7). Although there has been some regarding the relationship between LNM and prognosis, many studies have found that LNM is a risk factor for local tumor recurrence and cancer-specific mortality (8,9).
The gold standard for assessing LNM is based on lymph node biopsy or pathologic histologic examination after surgical lymph node dissection. However, such invasive methods often have certain adverse reactions, and patients without LNM can be checked excessively (10,11). Ultrasound (US) is a non-invasive and inexpensive technique that can be used to differentiate between benign and metastatic nodes (12).
However, the conventional US diagnostic criteria for malignant lymph nodes are still controversial, with low sensitivity and specificity (13). US imaging is operator dependent, and retropharyngeal, retrosternal, and mediastinum evaluations can be difficult (14-16). In contrast, computed tomography (CT) is a relatively objective imaging method. It has good spatial resolution, is not affected by the trachea or sternum, and is operator independent. CT can provide detailed, objective anatomic information with unlimited coverage, even to deep areas (16,17). Moreover, it can also provide detailed anatomic information for surgeons, particularly in terms of nodal locations and relationships to anatomical landmarks. However, it is easy to miss small lesions with CT (15), and it is currently not recommended as a routine imaging tool for thyroid malignancies (16). As far as we know, there is currently no evidence-based medical evidence for the diagnosis of LNM of TC by US + CT, and the results of various studies on its diagnostic efficacy are inconsistent (32–96% of sensitivity and 25–96% specificity) (8,11). Therefore, the diagnosis of cervical LNM by US + CT is controversial at present.
US and CT are both common and non-invasive examination techniques in daily medical work. It has been reported that US + CT is better than US alone in the diagnosis of cervical LNM (11,15), and clinically, many doctors tend to perform both US and CT examination in the diagnosis of cervical LNM. Meta-analysis is a research method superior to subjective judgment (18). It can overcome the shortage of small sample size and statistical strength, and carry out objective and quantitative comprehensive evaluation of research results (19). Suitable for solving the diagnostic study mentioned in this topic. In the present study, we discuss the diagnostic performance of this combination in the diagnosis of LNM in patients with TC. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-347/rc).
Methods
Study search
We performed a comprehensive search to identify studies that examined parallel test (1 of the 2 tests is positive) of US + CT for detecting and quantifying lymphatic metastases in TC. We searched PubMed, EMBASE, Cochrane library, Web of Science, and Wanfang Medical Network (Core journals only) using the following search criteria: “(ultrasound) OR (sonography) OR (color Doppler) AND (computed tomography) OR (CT) AND (lymph node) AND thyroid”.
We limited the search to titles and abstracts, and did not set date criteria. We included English- and Chinese-language publications. The bibliographies of relevant articles were also searched to identify any other relevant studies. The literature search and selection were performed independently by two radiologists.
Study selection
A study was included if it met the following criteria: (I) it evaluated the diagnostic performance of US + CT for LNM; (II) it involved patients with diagnosed TC, regardless of histopathology; (III) its reference standards were based on histopathologic tests; (IV) it clearly reported sensitivity and specificity, with 95% confidence intervals (CIs), or these could be calculated from 2×2 tables; (V) the full-text article was available; (VI) and the types of clinical design included prospective and retrospective studies.
Studies (or subsets) were excluded if any of the following criteria were met: (I) unsuitable publication types (e.g., case reports, conference proceedings, abstracts, reviews); (II) animal experiments or non-clinical studies; (III) incomplete data or without full text; (IV) overlapping studies (such as studies from the same study group, institution, or with the same results); (V) studies not focused on the diagnostic performance of US + CT for LNM detection, or CT-only detection; (VI) insufficient data for reconstruction of 2×2 tables; and (VII) studies lacking reference standards based on histopathologic tests.
For all cited articles, informed consent from each study participant and approval of the protocol by an ethics committee or institutional review board were obtained.
Quality assessment and data extraction
The methodologic quality of the included studies was independently assessed by 2 reviewers using tailored questionnaires and criteria provided by Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2). Any disagreements were minor and were resolved by consensus.
Two reviewers (Y Wang and M Chen) independently screened the literature and extracted data, including author, year, country, ethnicity, sample size, diagnostic criteria, outcome indicators (sensitivity, specificity, and number of true positives, false positives, false negatives, and true negatives). Missing data were supplemented by contacting the authors as needed. Quality assessment was based on the QUADAS-2 scale. QUADAS-2’s criteria for evaluating high-quality research include four stages: reporting review questions, developing review specific guidelines, reviewing published preliminary study flow charts, and determining bias and applicability (20). When there was disagreement, a third reviewer (G Yang) was consulted, and disagreement was settled through multilateral discussion.
Statistical analysis
Stata SE version 15.1 (StataCorp, College Station TX, USA) was used for the statistical analysis. Cochrane RevMan version 5.4 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman) was used for statistical analysis. P value of <0.05 was defined as statistical significance. Subgroup analysis and meta-regression analysis were performed using Meta-DiSc 1.4 (https://meta-disc.software.informer.com/1.4/). Sensitivity, specificity, the positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (DOR) were calculated. The area under the curve (AUC) was used for evaluating overall accuracy. The threshold effect was assessed using Spearman’s rank correlation coefficient (between the logic of sensitivity and logic of 1 − specificity). P<0.05 indicated a significant threshold effect.
Our primary outcome was the diagnostic performance of US + CT for the detection of cervical LNM in patients with TC. A secondary outcome was to clarify the parameters responsible for heterogeneity, by performing subgroup analyses according to the specific clinical settings.
Sensitivity and specificity, with 95% CIs, were calculated using bivariate random-effects modeling. When heterogeneity caused by a threshold effect was noted, it was analyzed visually; the results were graphically presented by plotting receiver-operating characteristic curves with 95% CIs and prediction regions. Deeks’ funnel plot was used to assess publication bias, and Deeks’ asymmetry test was used to calculate the P value for statistical significance.
Results
Document screening process and results
An initial systematic search identified 3,046 articles (PubMed, n=1,278; EMBASE, n=109; Cochrane, n=7; Web of Science, n=1,626; and other databases, n=26). We removed 2,537 studies (including duplicates, articles unrelated to the topic, conference abstracts, case reports, and conference proceedings) and screened the remaining 509 titles. We retained 35 studies and obtained their full text. After reviewing these, 24 articles were excluded. Finally, we included 11 studies in our meta-analysis (Figure 1).
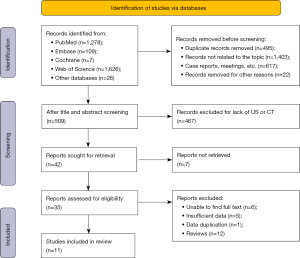
Inclusion of basic research characteristics
The basic characteristics of the present study are summarized in Table 1. Our meta-analysis included 11 eligible studies (10 English studies and 1 Chinese study) involving a total of 6,261 TC patients and 8,394 non-TC patients, with mean ages of 12–83 years, over a study period of 2008–2021 (8,11,21-29). Three of the studies were based in China and 8 in Korea. Two articles were on TC (25,27), while 9 focused on pathologically confirmed thyroid papillary carcinoma, a pathologic classification of TC. Pathologic diagnosis was used in all the included literature. One included study was prospective in design (27), whereas the others were retrospective. All the included studies evaluated the preoperative diagnostic performance of US + CT. All the studies used enhanced CT scanning, and almost all used conventional US techniques, including color Doppler and two-dimensional US. Eight of these articles used conventional US technology, including Doppler US; 2 did not mention the US technology used; and 1 mentioned the use of high-frequency US technology. The CT scanners used were heterogeneous. Four studies used only Siemens scanners; 1 study used only GE; 1 study used only Philips; 1 study used GE, Siemens, and Philips; and 4 studies did not specify the scanner. CT standards and cervical LNM US standards were inconsistent among the studies (Table 1). In 1 article (27), the US + CT diagnostic criteria did not include circular lymph nodes or the absence of fatty hilum in LNM; these were not considered to be fully confirmed radiographic features of LNM.
Table 1
| First author | Year | Country | Age (years), mean ± SD | Patients (n) | C | L | Timing of imaging | Histologic type | Study design | CT equipment | US equipment | CT technology | Suspicious CT features | US technology | Suspicious US features | Patient enrollment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ying Liu | 2021 | China | 40.5±9.3 | 600 | 600 | NA | Pre | PTC | R | Philips | GE/Siemens | Contrast enhanced | Enhancement, heterogeneous enhancement, calcification, or cystic or necrotic change | Conventional | Focal or diffuse hyper-echogenicity, calcification, cystic change, abnormal vascular pattern, round shape | NA |
| So Yeon Yang | 2020 | Korea | 45±31 | 453 | 453 | NA | Pre | PTC | R | Siemens | Philips | Contrast enhanced | Strong enhancement without hilar vessel enhancement, heterogeneous enhancement, calcification, or cystic or necrotic change | Conventional | Focal or diffuse hyper-echogenicity, microcalcification, cystic change, abnormal vascular pattern, round shape | Consecutive |
| Younghen Lee | 2018 | Korea | 47.1±12.3 | 351 | 531 | 263 | Pre | TC | P | NA | NA | Contrast enhanced | Calcification, cystic change, strong enhancement without hilar vessel enhancement, heterogeneous enhancement | Conventional | Calcification, cystic change, hyper-echogenicity, peripheral or chaotic color Doppler pattern | Consecutive |
| Qiaoqiao Wei | 2018 | China | NA | 69 | NA | NA | Pre | PTC | R | Siemens | Siemens | Contrast enhanced | NA | Conventional | Local or diffuse high-level echo, small or coarse calcification, cystic degeneration, and sub-round shape | Random |
| Seo Ki Kim | 2017 | Korea | NA | 3,668 | 6,577 | NA | Pre | PTC | R | NA | NA | Contrast enhanced | NA | NA | NA | NA |
| Dae Kwon Na | 2015 | Korea | 48.5±25.5 | 176 | 176 | 176 | Pre | TC | R | GE | GE | Contrast enhanced | Strong enhancement without hilar vessel enhancement, heterogeneous enhancement, calcification, or cystic or necrotic change | High frequency | Irregular cystic change, microcalcification, focal or diffuse hyper-echogenicity, round shape, and loss of fatty echogenic hilum | NA |
| Ganxun Wu | 2014 | China | 44±32 | 115 | 110 | 141 | Pre | PTC | R | NA | NA | Contrast enhanced | Calcification, heterogeneity, cystic change, enhancement | Conventional | Calcification, heterogeneity, cystic change, enhancement, round shape, abnormal vascular pattern | NA |
| DW Lee | 2013 | Korea | 48.5±33.5 | 252 | 262 | 148 | Pre | PTC | R | NA | NA | NA | Enhancement, heterogeneity, calcification, cystic change, round shape (did not apply size criteria for lymph node metastases at level VI) | NA | Enhancement, heterogeneity, calcification, cystic change, round shape (did not apply size criteria for lymph node metastases at level VI) | NA |
| Jung Hyun Yoon | 2011 | Korea | 49±34 | 113 | NA | 122 | Pre | PTC | R | Siemens | Philips | Contrast enhanced | Calcification, central necrosis or cystic change, heterogeneous cortical enhancement | Conventional | Hyper-echogenicity, loss of fatty hilum, cystic change, calcification, round shape, abnormal vascular pattern | Consecutive |
| Ji Soo Choi | 2009 | Korea | 47±27 | 299 | 299 | 53 | Pre | PTC | R | Siemens | Philips | Contrast enhanced | Calcification, cystic or necrotic change, heterogeneous enhancement, strong enhancement without hilar vessel enhancement | Conventional | Focal or diffuse hyper-echogenicity, microcalcification, cystic change, and abnormal vascular pattern | NA |
| Eunhee Kim | 2008 | Korea | 47±31 | 165 | 133 | 144 | Pre | PTC | R | GE/Siemens/Philips | Philips | Contrast enhanced | Strong enhancement without hilar vessel enhancement, heterogeneous enhancement, calcification, or cystic or necrotic change | Conventional | Focal or diffuse hyper-echogenicity, calcification, cystic change, abnormal vascular pattern, or a round shape | Consecutive |
C, central cervical lymph node metastasis; CT, computed tomography; L, lateral cervical lymph node metastasis; NA, not available; P, prospective; Pre, preoperative; PTC, papillary thyroid cancer; R, retrospective; TC, thyroid cancer; US, ultrasound.
Methodologic quality evaluation
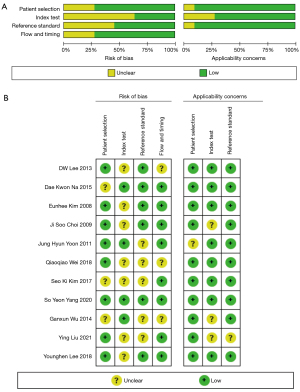
Figure 2 shows the methodologic quality assessment. Most of the risk assessments included in the study were low risk, with no high-risk items. Therefore, the offset risk level included in the study has little influence on the meta-analysis aggregate effect. The methodologic quality of the included studies was independently assessed by 2 reviewers using tailored questionnaires and QUADAS-2 criteria. Disagreements were minor and were resolved by consensus.
US + CT application to central cervical LNMs
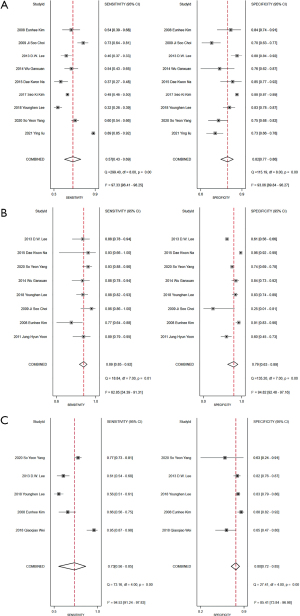
Nine studies (including 6,079 patients and 9,289 compartments) were pooled and analyzed to determine their diagnostic performance for central cervical LNMs. Sensitivity and specificity of the individual studies were 43–69% and 77–86%, respectively. The Higgins I2 statistic showed significant heterogeneity in terms of sensitivity (I2=97.33%) and specificity (I2=93.06%). The summary sensitivity and specificity of US + CT for diagnosing central LNMs were 0.57 (0.43–0.69) and 0.82 (0.77–0.86), respectively (Figure 3A).
US + CT application to lateral cervical LNMs
Eight studies (including 1,924 patients and 1,802 compartments) were pooled and analyzed to determine their diagnostic performance for lateral cervical LNMs. Sensitivity and specificity of the individual studies were 85–92% and 63–89%, respectively (Figure 3B). The Higgins I2 statistic showed significant heterogeneity in terms of sensitivity (I2=62.85%) and specificity (I2=94.82%). Significant heterogeneity among studies was observed in the summary sensitivity and specificity. The summary sensitivity and specificity of US + CT for diagnosing lateral LNMs were 0.89 (0.85–0.92) and 0.79 (0.63–0.89), respectively.
US + CT application to all compartments of cervical LNMs
Five studies (including 1,314 patients and 2,037 compartments) were pooled and analyzed to determine their diagnostic performance for all compartments of cervical LNMs. Pooled sensitivity was 0.73 (95% CI: 0.56–0.85, I2=94.53%) and pooled specificity was 0.80 (95% CI: 0.72–0.85, I2=85.41%) (Figure 3C). The pooled DOR was 11 (95% CI: 6–18, I2=83.8%) and the AUC was 0.83 (95% CI: 0.80–0.86). Figure 4 shows the receiver operation characteristic curve for US + CT diagnosis of cervical LNM.

Heterogeneity exploration of the threshold effect
The pooled analysis indicated significant heterogeneity. Spearman’s rank correlation coefficient (to assess the threshold effect) in the central, lateral, and all compartments was 0.767 (P=0.016), 0.381 (P=0.352), and 0.600 (P=0.285), respectively, indicating the existence of threshold effect.
Assessment of publication bias
As shown in the three Deeks’ funnel plot (Figure 5), there were no significant publication biases among the included studies.

Meta-regression analysis
Our meta-regression analysis is shown in Table 2. Seven factors might have contributed to heterogeneity. We performed subgroup analyses for central and lateral cervical lymph nodes separately (Table 2). We also analyzed potential covariates (e.g., study design, country, historic type, sample size, and region). High heterogeneity was indicated by a Q-test P<0.05. We concluded that the high heterogeneity of cervical LNM diagnosis could be related to lymph node location (P=0.0075). The heterogeneity of diagnosis in the central region was not related to study design, country, historic type, sample size, or patient enrollment (P>0.005). P values <0.05 were considered statistically significant.
Table 2
| Covariate | Subgroup | Sensitivity | Specificity | P value |
|---|---|---|---|---|
| Central compartments | Total | 0.519 | 0.859 | |
| Consecutive patients | Yes | 0.485 | 0.809 | 0.1900 |
| No | 0.523 | 0.865 | ||
| Study design | R | 0.529 | 0.861 | 0.2663 |
| P | 0.324 | 0.830 | ||
| Country | Korea | 0.483 | 0.866 | 0.7984 |
| China | 0.825 | 0.734 | ||
| Historic type | PTC | 0.532 | 0.861 | 0.2487 |
| TC | 0.337 | 0.835 | ||
| Analysis | Patient based | 0.725 | 0.744 | 0.5404 |
| Node based | 0.471 | 0.871 | ||
| Sample size | ≥200 | 0.521 | 0.860 | 0.6099 |
| <200 | 0.477 | 0.826 | ||
| Lateral compartments | Total | 0.890 | 0.752 | |
| Consecutive patients | Yes | 0.885 | 0.767 | 0.9470 |
| No | 0.900 | 0.735 | ||
| Study design | R | 0.893 | 0.745 | 0.8451 |
| P | 0.881 | 0.827 | ||
| Country | Korea | 0.892 | 0.747 | 0.8760 |
| China | 0.877 | 0.838 | ||
| Historic type | PTC | 0.892 | 0.708 | 0.3677 |
| TC | 0.884 | 0.910 | ||
| Analysis | Patient based | 0.929 | 0.963 | 0.1523 |
| Node based | 0.889 | 0.719 | ||
| Sample size | ≥200 | 0.899 | 0.699 | 0.7181 |
| <200 | 0.876 | 0.870 | ||
| Region | Central | – | – | 0.0075 |
| Lateral | – | – |
P, prospective; PTC, papillary thyroid cancer; R, retrospective; TC, thyroid cancer.
Discussion
US is the first line of examination for planning initial and subsequent surgery in patients with suspected or confirmed thyroid malignancies (11). However, in daily medical work, we rarely perform US examination alone for diagnosis, as imaging alone is not sufficient to guide clinical staging and treatment decisions for TC patients. In this context, CT has emerged as a relatively convenient and optimized examination method that can overcome the anatomical limitations and operator dependency of US. There have been previous meta-analyses that compared US and CT for diagnosing LNM (30,31). However, to the best of our knowledge, there are no published meta-analyses on the diagnostic efficacy of US + CT, which is the focus of the present study.
We investigated the diagnostic performance of US + CT for the assessment of cervical LNM in TC patients by pooling 11 individual studies that included 6,461 patients. The pooled sensitivity, specificity, and AUC of US were 0.57, 0.82, and 0.80, respectively. The pooled sensitivity, specificity, and AUC for lateral cervical LNM were 0.89, 0.79, and 0.91, respectively. Analysis of heterogeneity among the included studies showed a threshold effect for diagnosing LNM in the central region. Deeks’ metrics test, based on the funnel plot, showed that there was no significant publication bias in terms of cervical LNM diagnosis using US + CT in TC patients.
Compared with US alone or CT alone, US + CT is relatively sensitive and less specific in diagnosing both central and lateral cervical lymph nodes. Previous studies have shown that US has high specificity for diagnosing cervical lymph nodes, but poor accuracy for the detecting of LNMs in the central region of the neck (31). CT has higher sensitivity than US for diagnosing central lymph nodes, but CT has a lower specificity than US for diagnosing central lymph nodes and the contralateral neck (28,29,31).
We also compared other diagnostic imaging techniques. Cho et al. reported that magnetic resonance imaging (MRI) had a pooled sensitivity of 80% (95% CI: 68–88%) and a pooled specificity of 85% (95% CI: 63–95%) for diagnosing cervical LNM in TC patients (32). Compared with MRI, which is also free of ionizing radiation, US + CT could be slightly less sensitive and specific. However, MRI is more expensive and time consuming than US + CT. Contrast-enhanced US is a novel imaging method to characterize superficial LNM, and has high sensitivity and specificity. According to Hong et al. (33), contrast-enhanced US alone might not be as effective as conventional US for the diagnosis of LNMs; the AUC for conventional US plus contrast-enhanced US is higher that of contrast-enhanced US alone.
LNM in the central region, lateral neck, and whole neck were analyzed. The data showed that US + CT had a high heterogeneity of diagnosis in the central and cervical regions, but low heterogeneity in the contralateral cervical region. We performed a meta-regression analysis of potentially related parameters. However, the high heterogeneity of the central region could not be attributed to any parameter. We hypothesize that the heterogeneity could be related to the complex anatomical structure of the central cervical region, the large difference in diagnostic results from the central lymph nodes from different doctors, and equipment quality and imaging resolution, which could also affect the diagnosis. According to our analysis, the reason for diagnostic heterogeneity in LNM of the whole neck is related to the location of lymph nodes (P=0.0075).
LNM diagnostic criteria differ. Yoon et al. (22) recommended diagnosis based on a round shape or loss of fatty hilum, because these features, without any other suspicious features, are not fully validated for LNM. Moreover, similar effects are caused by lymph node tuberculosis, lymphoma, and reactive proliferative lymph nodes. Because published research is limited to mostly single-center studies, further randomized, blind, large-sample, multicenter studies are required for evaluating uniform US and CT imaging features of metastatic lymph nodes.
Our study has some limitations. First, the number of articles we searched was low, and most of them evaluated the effectiveness of preoperative diagnosis. Second, most of these studies were retrospective, and we cannot rule out the possibility of residual confounding variables and the possibility that some lymph nodes might not have been properly diagnosed. Third, most of the studies did not specify whether they were blinded. Finally, most of the studies did not specify the interval between examination and postoperative pathologic results, and all were single-center studies.
The diagnostic efficiency of CT for lateral cervical LNM is greater than for central cervical LNM. CT has high sensitivity and accuracy for the diagnosis of central cervical LNMs. US + CT is important for the preoperative examination of cervical LNMs in TC.
Acknowledgments
Funding: This work was supported by the Agriculture and Social Development Plan of Hangzhou (grant No. 20190101A09) and the Medical Science and Technology Project of Zhejiang Province (grant No. 2021KY911).
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-347/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-347/coif). All authors report that this work was supported by the Agriculture and Social development plan of Hangzhou (grant No. 20190101A09) and the Medical Science and Technology Project of Zhejiang Province (grant No. 2021KY911). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Prete A, Borges de Souza P, Censi S, et al. Update on Fundamental Mechanisms of Thyroid Cancer. Front Endocrinol (Lausanne) 2020;11:102. [Crossref] [PubMed]
- Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the Management of Thyroid Cancer. Horm Metab Res 2020;52:562-77. [Crossref] [PubMed]
- Choi YJ, Yun JS, Kook SH, et al. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg 2010;34:1494-9. [Crossref] [PubMed]
- Marshall CL, Lee JE, Xing Y, et al. Routine pre-operative ultrasonography for papillary thyroid cancer: effects on cervical recurrence. Surgery 2009;146:1063-72. [Crossref] [PubMed]
- Touboul C, Piel B, Koskas M, et al. Factors predictive of endometrial carcinoma in patients with atypical endometrial hyperplasia on preoperative histology. Anticancer Res 2014;34:5671-6. [PubMed]
- Cuperjani F, Gashi L, Kurshumliu F, et al. Relationship between Ribosomal Protein S6-pS240 Expression and other Prognostic Factors in Non-Special Type Invasive Breast Cancer. Breast Care (Basel) 2019;14:171-5. [Crossref] [PubMed]
- Kim E, Park JS, Son KR, et al. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008;18:411-8. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Zhou J, Zhang B, Dong Y, et al. Value on the diagnosis of axillary lymph node metastasis in breast cancer by color Doppler ultrasound combined with computed tomography. J BUON 2020;25:1784-91. [PubMed]
- Liu Y, Li S, Yan C, et al. Value of dual-phase, contrast-enhanced CT combined with ultrasound for the diagnosis of metastasis to central lymph nodes in patients with papillary thyroid cancer. Clin Imaging 2021;75:5-11. [Crossref] [PubMed]
- Dietrich CF, Ponnudurai R, Bachmann Nielsen M. Is there a need for new imaging methods for lymph node evaluation? Ultraschall Med 2012;33:411-4. [Crossref] [PubMed]
- Wensing BM, Merkx MA, De Wilde PC, et al. Assessment of preoperative ultrasonography of the neck and elective neck dissection in patients with oral squamous cell carcinoma. Oral Oncol 2010;46:87-91. [Crossref] [PubMed]
- Moon HJ, Kim EK, Yoon JH, et al. Differences in the diagnostic performances of staging US for thyroid malignancy according to experience. Ultrasound Med Biol 2012;38:568-73. [Crossref] [PubMed]
- Lesnik D, Cunnane ME, Zurakowski D, et al. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 2014;36:191-202. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Yi KH. The Revised 2016 Korean Thyroid Association Guidelines for Thyroid Nodules and Cancers: Differences from the 2015 American Thyroid Association Guidelines. Endocrinol Metab (Seoul) 2016;31:373-8. [Crossref] [PubMed]
- Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med 2018;33:277-83. [Crossref] [PubMed]
- Walker E, Hernandez AV, Kattan MW. Meta-analysis: Its strengths and limitations. Cleve Clin J Med 2008;75:431-9. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Choi JS, Kim J, Kwak JY, et al. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol 2009;193:871-8. [Crossref] [PubMed]
- Yoon JH, Kim JY, Moon HJ, et al. Contribution of computed tomography to ultrasound in predicting lateral lymph node metastasis in patients with papillary thyroid carcinoma. Ann Surg Oncol 2011;18:1734-41. [Crossref] [PubMed]
- Lee DW, Ji YB, Sung ES, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol 2013;39:191-6. [Crossref] [PubMed]
- Wu G, Chen W, Yang L, et al. The combination of ultrasound and CT evaluate lymph node metastasis of thyroid papillary carcinoma in different compartments. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014;28:252-5. [PubMed]
- Na DK, Choi YJ, Choi SH, et al. Evaluation of cervical lymph node metastasis in thyroid cancer patients using real-time CT-navigated ultrasonography: preliminary study. Ultrasonography 2015;34:39-44. [Crossref] [PubMed]
- Kim SK, Woo JW, Park I, et al. Computed Tomography-Detected Central Lymph Node Metastasis in Ultrasonography Node-Negative Papillary Thyroid Carcinoma: Is It Really Significant? Ann Surg Oncol 2017;24:442-9. [Crossref] [PubMed]
- Lee Y, Kim JH, Baek JH, et al. Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck 2018;40:2137-48. [Crossref] [PubMed]
- Wei Q, Wu D, Luo H, et al. Features of lymph node metastasis of papillary thyroid carcinoma in ultrasonography and CT and the significance of their combination in the diagnosis and prognosis of lymph node metastasis. J BUON 2018;23:1041-8. [PubMed]
- Yang SY, Shin JH, Hahn SY, et al. Comparison of ultrasonography and CT for preoperative nodal assessment of patients with papillary thyroid cancer: diagnostic performance according to primary tumor size. Acta Radiol 2020;61:21-7. [Crossref] [PubMed]
- Cho SJ, Suh CH, Baek JH, et al. Diagnostic performance of CT in detection of metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Eur Radiol 2019;29:4635-47. [Crossref] [PubMed]
- Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol 2019;112:14-21. [Crossref] [PubMed]
- Cho SJ, Suh CH, Baek JH, et al. Diagnostic performance of MRI to detect metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Clin Radiol 2020;75:562.e1-562.e10. [Crossref] [PubMed]
- Hong YR, Luo ZY, Mo GQ, et al. Role of Contrast-Enhanced Ultrasound in the Pre-operative Diagnosis of Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Carcinoma. Ultrasound Med Biol 2017;43:2567-75. [Crossref] [PubMed]
(English Language Editor: R. Scott)


