
Risk factors for chest wall depression after implant insertion for breast reconstruction: a retrospective quantitative study
Introduction
Breast cancer is one of the most common malignancies in women (1). Along with the increase in its incidence, mastectomy rates have also largely increased in the past decade. In Korea, breast reconstruction after total mastectomy has been covered by the National Health Insurance Service since April 2015. Since this coverage, the number of patients undergoing reconstruction has significantly increased. In both the United States and Korea, implant-based reconstruction represents the most common form of breast reconstruction after mastectomy (2). Considering the increasing number of surgeries using implants, many efforts have been made to reduce implant-related complications. The use of the subpectoral plane could cover the implant with well-vascularized muscles and effectively prevent implant extrusion or skin flap necrosis (3). However, a significant number of patients have been reported to develop some degree of animation deformity, i.e., visible contraction and lateral displacement of the breast implant (4). In addition, patients often experience severe postoperative pain caused by detachment of the pectoralis major muscle and muscular expansion (5). The use of acellular dermal matrices (ADM) for implant wrapping and pocket definition has decreased rates of implant-related complications, including capsular contracture and skin flap compromise (6,7). Currently, breast surgeons can achieve well-vascularized thicker flaps as comprehending the fascia system of the breast (8). The complication rate of prepectoral implant reconstruction was significantly lowered by applying these advances; however, despite the lowered complication rate, implant-related complications such as capsular contracture and implant malposition are still a problem. Although many studies have reported complications related to implants, there have been few reports discussing chest wall deformation following implant insertion. Some studies have described cases of chest wall deformity due to the tissue expander that is used for breast reconstruction (9,10). These reports suggested that the chest wall could be deformed or depressed after maximal expansion of the tissue expander. However, we observed some cases of chest wall depression (CWD) that occurred after direct-to-implant (DTI) breast reconstruction despite no pressure caused by the tissue expander. Therefore, we assumed that CWD could also occur after DTI breast reconstruction. The aim of this study was to quantify CWD after breast implant insertion and identify possible risk factors. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-101/rc).
Methods
Between January 2017 and December 2020, patients undergoing mastectomy were enrolled at our institution. A retrospective chart review was performed, identifying all patients who underwent prepectoral or subpectoral DTI breast reconstruction. This study was approved by the Institutional Review Board of the committee. Patients who underwent unilateral DTI reconstruction without contralateral augmentation mammoplasty were included in the study. All cases were performed by a single senior surgeon (CYH) from 2017 to 2020. CWD was measured in patients who underwent a computed tomography (CT) scan at >12 months after the DTI surgery. Exclusion criteria consisted of failed implant-based reconstruction during the follow-up period, tissue expander-based reconstruction, and delayed reconstruction or autologous-based reconstruction. Patients who underwent additional surgery due to cancer recurrence were also excluded. Patient demographics (age and body mass index), implant size, inserted plane (subpectoral or prepectoral), postmastectomy radiation therapy, and chemotherapy were assessed. Postoperative complications including infection, seroma, and hematoma were recorded. Capsular contracture was assessed by an experienced surgeon (CYH) based on the Baker grade. Grade I is considered as no capsular contracture and Grade II was determined only when the contractures are definitely different from Grade I on palpation. Grade III and IV were diagnosed when there was an apparent change on visual examination. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Seoul National University Bundang Hospital (No. B-2107-698-104) and informed consent was waived in view of the retrospective nature of the study.
Surgical techniques
Mastectomy was performed through an inframammary incision, a lateral S-shaped incision, or a radial incision, and skin flaps were elevated along the prepectoral plane. Clinical evaluation of the skin flaps was carefully performed using indocyanine green fluorescence imaging. Patients were categorized into the dual plane submuscular group or prepectoral group based on the surgeon’s intraoperative assessment. In prepectoral reconstruction, the implant was wrapped entirely in ADM. The superior, medial, lateral, and inferior borders of this ADM envelope were then fixed with absorbable sutures. In subpectoral placement, the pectoralis major muscle was elevated to create the subpectoral pocket, and the inferior margin of the muscle was divided. The ADM was used to provide coverage for the inferior pole of the implant. Subpectoral DTI is performed when mastectomy flap is thin and there is a possibility of related complications such as ischemia of the mastectomy flap or capsular contracture. Intraoperatively, the pectoralis major muscle was sufficiently released from its insertion and the ADM was widely applied to cover the inferior pole for minimizing complications including capsular contracture or animation deformity. In all patients, smooth-surfaced, round implants manufactured by BellaGel® or Mentor® were used.
Chest wall deformity index (CDI) analysis
We defined ‘L1 and L2’ as the maximal length between the anterior and posterior walls; ‘L1 and L2’ were defined as the length of the chest wall on the contralateral side and the operated side, respectively. This length was the distance of the perpendicular line drawn from the most anterior border of the fourth rib bone to the most posterior point of the chest wall; boundaries of the chest wall were defined as intercostal muscle and costal bone and cartilage (Figure 1). The CT was taken with the patient in a supine position, and as shown in Figure 1, the midline connecting the spine and the sternum was set as the vertical axis and the antero-posterior (AP) length was measured with a straight line parallel to this line. Preoperative (L1, L2) and postoperative (P1, P2) lengths were recorded. There was a difference in the AP length of the chest wall between the inspiratory and expiratory CT scans. Therefore, we used a CWD ratio (CDR) to measure the change in AP length. Pre- and post-CDR were defined as L2/L1 and P2/P1, respectively. CDI was defined as post-CDR/pre-CDR to correct for natural differences between both chest walls.

Statistical analysis
The pre- and postoperative difference of AP length was analyzed using a paired t-test. Comparisons between groups were performed using the χ2 test for categorical variables and Mann-Whitney U test for quantitative variables. Univariate analysis was used to examine the correlation of each variable with pre- and post-CDI. Comparisons of categorical variables were performed using Mann-Whitney test or Student’s t-test, depending on whether the data were normally distributed. Continuous variables were analyzed using the Spearman rank correlation test. We also analyzed correlation and collinearity between variables. Multivariate analysis was achieved using a stepwise multiple linear regression with factors that showed P value of <0.2 in the univariate analyses to identify factors associated with pre- and post-CDI. Statistical analysis was performed using IBM SPSS Version 24 (IBM Corp., Armonk, NY). Statistical significance was set at P value of <0.05.
Results
A total of 57 patients underwent immediate reconstruction by a single senior surgeon (CYH) and the mastectomies were performed by a single oncologic surgeon (EKK). The demographic data of the patient populations who underwent implant-based reconstruction are analyzed (Table 1). The mean patient age was 44.5 years (range, 32–65 years), the mean body mass index was 22.1 kg/m2 (range, 17.6–31.6 kg/m2), and the average implant size was 252.4 cc (range, 125–550 cc). Adjuvant radiotherapy (RT) and chemotherapy were performed in 23 and 20 patients, respectively. All patients underwent nipple sparing mastectomy since two-stage reconstruction was performed using a tissue expander in the case of skin sparing mastectomy. Of the 57 patients, 42 underwent implant reconstruction using the prepectoral plane and 15 had subpectoral implant reconstruction. The mean follow-up period was 21.4 months (range, 12–45 months). One patient showed postoperative infection and the hematoma evacuation procedure was performed in two patients (Table 2).
Table 1
| Characteristics | Value |
|---|---|
| No. of patients (%) | 57 (100.0) |
| Age, years | |
| Mean | 44.5±6.9 |
| Range | 32–65 |
| BMI, kg/m2 | |
| Mean | 22.1±3.2 |
| Range | 17.6–31.6 |
| Adjuvant radiotherapy, n (%) | 23 (40.4) |
| Chemotherapy, n (%) | 20 (35.1) |
| Adjuvant | 14 (24.6) |
| Neoadjuvant | 6 (10.5) |
| Follow-up period (months) | |
| Mean | 21.4±6.7 |
| Range | 12–45 |
BMI, body mass index.
Table 2
| Characteristics | Value |
|---|---|
| Total No. of patients (%) | 57 (100.0) |
| Type of mastectomy, n (%) | |
| Nipple-sparing mastectomy | 57 (100.0) |
| Inserted plane, n (%) | |
| Prepectoral | 42 (73.7) |
| Subpectoral | 15 (26.3) |
| Implant | |
| Size (cc), median [range] | 252.4 [125–550] |
| Mentor®/Bellagel® | 28/29 |
| Capsular contracture (Baker grade), n (%) | |
| I | 35 (61.4) |
| II | 19 (33.3) |
| III | 3 (5.3) |
| Postoperative complications, n (%) | |
| Infection | 1 (1.8) |
| Hematoma | 2 (3.5) |
The averages of L1, L2, L1’, and L2’ were 165.1 (range, 145–200), 165.2 (range, 140–199), 163.0 (range, 142–182), and 158.9 (range, 132–181) mm, respectively. Pre- and post-CDR were 1.001 (range, 0.873–1.097) and 0.975 (range, 0.871–1.058), respectively. The difference between pre- and post-CDR was statistically significant in the paired t-test (P<0.001). There were no functional problems such as rib fracture or dyspnea. The CDI (post-CDR/pre-CDR) was 0.975 (range, 0.917–1.012). The mean of the preoperative AP length multiplied by (1-CDI) was 4.16 mm (range, −2.16 to 13.82 mm), which represents the average depth of depression. In univariate analysis, age, radiation therapy, and capsular contracture were significant factors related to CWD (Tables 3,4). In multivariate analysis, capsular contracture was the independent prognostic factor, which was positively correlated with CDI (P=0.041). Likewise, patients’ age was the independent prognostic factor showing negative correlation in the multivariate analysis (P=0.015) (Table 5).
Table 3
| Variables | No. | CDI | p |
|---|---|---|---|
| RT | |||
| RT (−) | 34 | 0.9805±0.0200 | 0.012* (t-test) |
| RT (+) | 23 | 0.9656±0.0231 | |
| CT | |||
| CT (−) | 37 | 0.9751±0.0216 | 0.775 (t-test) |
| CT (+) | 20 | 0.9734±0.0242 | |
| Plane | |||
| Prepectoral | 42 | 0.9758±0.0230 | 0.481 (t-test) |
| Subpectoral | 15 | 0.9710±0.0209 | |
| Capsular contracture (Baker grade) | |||
| − (I) | 35 | 0.9810±0.0178 | 0.009* (MW test) |
| + (II and III) | 22 | 0.9641±0.0253 | |
*, statistically significant (P<0.05). CDI, chest wall deformity index; RT, radiotherapy; CT, chemotherapy; MW test, Mann-Whitney test.
Table 4
| Variables | r | P |
|---|---|---|
| BMI | 0.049 | 0.417 |
| Age | 0.482 | 0.001* |
| Implant size | −0.066 | 0.628 |
| Follow up | −0.231 | 0.084 |
r, Spearman rank correlation coefficient; *, statistically significant (P<0.05). CDI, chest wall deformity index; BMI, body mass index.
Table 5
| Variables | β | SE | stdβ | t | P |
|---|---|---|---|---|---|
| Age | 0.001 | 0.000 | 0.345 | 2.522 | 0.015* |
| Capsular contracture | −0.011 | 0.005 | −0.252 | −2.093 | 0.041* |
| Radiotherapy | −0.006 | 0.006 | −0.123 | −0.906 | 0.369 |
| Follow up | −0.001 | 0.000 | −0.196 | −1.662 | 0.103 |
R2=0.339, adjusted R2 =0.298, P<0.001. *, P<0.05. R2, coefficient for determination. β, partial regression coefficient; SE, standard error; stdβ, standardized partial regression coefficient.
Discussion
To the best of our knowledge, this study is the first to report the possibility of CWD in patients who undergo DTI insertion unilaterally rather than implant insertion after the expander. In this study, capsular contracture and age were identified as factors for the development of CWD. The CDI was 0.975 and average depth of CWD after implant insertion was 4.16 mm. In addition, the difference of AP length was statistically significant.
Some case reports have described cases of CWD following tissue expansion for breast reconstruction (9,10). They presumed that pressure effects of tissue expansion caused rib fractures of the anterior thorax or chest wall contour changes. Sinow et al. observed costal and CWD in 81% and 68% of patients undergoing expander insertion, respectively (10). In this study, early capsular contracture was negatively correlated with CWD and they noted that the capsule itself acts as a buffer against deformation.
Cherubino et al. reported that deformities of the chest wall were caused by the use of tissue expanders (11). In this study, 22 of 36 patients showed remaining deformities at the 1-year follow-up after tissue expander removal and implant insertion. However, there were no significant risk factors that affected the integrity of the chest wall. Makiguchi et al. reported that CWD is common after tissue expander insertion and after maximal expansion of the tissue expander for breast reconstruction (12). However, there have been no studies on the possibility of CWD following implant insertion. In the case of aesthetic breast augmentation, it is difficult to identify the degree of deformity because implants are inserted bilaterally. Therefore, this assessment can be performed only in the case of unilateral breast reconstruction. As in the studies by Cherubino et al. and Makiguchi et al., when implants are inserted after tissue expander removal, it is difficult to observe the effect of only the implant because the effect during the expansion period cannot be excluded. On the contrary, the CWD may tend to improve due to the recoiling phenomenon after expander removal (11,12). The long-term results are insufficient to confirm CWD after DTI procedure because DTI has recently become available with an appropriate mastectomy flap and use of ADM (13). However, we observed some cases of CWD when we changed breast implants due to capsular contracture or patient requests. Based on these cases, we hypothesized that a similar phenomenon could occur even after DTI breast reconstruction.
Capsular contracture was positively correlated with CDI and patients’ age was negatively correlated with CDI, meaning that the presence of capsular contracture and a younger age increased the degree of CWD. There have been studies reporting osteoporosis as a risk factor for CWD and it was assumed that the risk of depression may increase because the prevalence of osteoporosis increases with older age (9,14). However, the opposite result was observed in our study. We hypothesize two theories for these results. First, there is a possibility that the cartilaginous portion of the rib was depressed due to constant pressure from the implants. This assumption came from some patients who underwent implant change, in whom CWD was seen mainly in the cartilaginous portion. It is well-known that cartilage tissue undergoes remodeling, degradation, and deformation due to the effects of mechanical loading (15). Previous biomechanical studies suggested that calcification may have a significant effect on the stiffness of the costal cartilage and the lengthwise extent of calcified regions of cartilage segments is seen to increase significantly with age (16,17). This age-related calcification could influence the results of the reduced depression. Second, older patients are more likely to have loose and ptotic breasts, which may have the effect of changing or dispersing the vector of pressure induced by the implant to the chest wall.
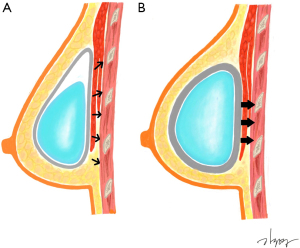
Capsular contracture was a significant predictor of CWD in the study by Makiguchi et al. (12). Contrary to previous studies, they reported that capsular contracture could induce higher pressure and had a positive correlation with deformity when the expander was in an overinflated state. However, in our study, despite the absence of inflation-induced pressure, the degree of CWD was more significantly observed in the presence of capsular contracture. We hypothesized that depression is more likely to occur when constant pressure is applied to the same area. In this case, capsular contracture can induce the effect of constant pressure to one point by holding the implant in one place (Figure 2). Capsular contracture can occur in the absence of RT, but many studies have indicated that postoperative RT worsened the degree of capsular contracture, as fibrosis is known to be a late normal tissue effect resulting from RT (18). In this study, among capsular contracture grades II and III patients, the proportion of patients receiving RT was 14 out of 22 (63.6%) and the positive correlation between RT and capsular contracture was also statistically significant (P<0.05, chi-squared test). However, in the case of patients undergoing RT, there may be a bias in evaluating low-grade capsular contracture due to changes in soft tissue, such as volume reduction and contracture following postoperative RT (19). The Baker grading system is a subjective classification based on clinical findings consisting of hardness and shape (20). In the case of soft tissue firmness after RT, it is difficult to clearly distinguish it from capsular contracture, so care must be taken in interpreting the presence of capsular contracture in this study. Therefore, we cannot exclude the possibility that CWD was independently affected by RT due to decreased soft tissue elasticity or volume. However, in most cases of capsular contracture that has progressed to some degree, an experienced breast surgeon can distinguish it from the difference caused by soft tissue change after RT. We performed multivariate analysis including these factors because the variance inflation factor between factors was sufficiently low despite a statistically significant correlation. Although RT was a significant factor in univariate analysis, it is a non-significant factor in multivariate analysis, so it is assumed that the capsular contracture induced by RT affects CWD rather than RT itself. Some studies have reported that the possibility of capsular contracture increases when RT is followed by subpectoral DTI (21,22). However, there was no significant difference of capsular contracture in our cohort; this difference, as mentioned in the surgical technique, seems to result from sufficient release of the pectoralis muscle for minimizing complications, including animation deformity.
The mean of the preoperative AP length multiplied by (1-CDI) was 4.16 mm, representing the average depth of depression. A depression of ≤5 mm is usually not noticeable and does not cause functional problems. The height of implants used for breast reconstruction in Asian countries is usually lower than that in Western countries. In most cases (54/57) in our study, the height of the implants was 30–50 mm. In this case, if the depression is >10 mm, it can cause discernible asymmetry, so it is necessary to consider the risk factors for such depression. In addition, implant-based breast reconstruction is often performed with contralateral augmentation mammoplasty in Korea (2). In these cases, the CWD itself can be a factor that makes the asymmetry more prominent, so it is necessary to consider this risk in advance. Therefore, we should inform the possibility of such asymmetry to the younger patients who are scheduled for postoperative RT.
There are several limitations to our study. First, since a relatively small number of patients were included and the follow-up period was not long, a long-term study with a larger number of patients is needed. It was important to measure the AP length of the chest wall in a consistent postoperative follow-up period, because postoperative chest wall change does not progress like a linear function graph. We had to analyze with 1-year postoperative CT scan; however, this short follow-up period may not be sufficient to observe capsular contracture, which is a limitation of this study. There is the possibility that longer-term follow-up of over 5 years can identify other risk factors for CWD such as follow-up period and implant size. Although the measurement of the AP length was performed with the vertical axis aligned, there may be small errors in patients with scoliosis. Another limitation is the retrospective nature of the analysis. We expect that further larger studies with a prospective design could overcome these limitations.
Conclusions
In conclusion, this is the first study showing the possibility of CWD following implant insertion. We also determined that the presence of capsular contracture and younger age increased the risk of CWD. Plastic surgeons should be aware of the possibility and risk factors of CWD following implant insertion to better inform patients.
Acknowledgments
The authors would like to thank Kyu-Yeong Kim for his expert assistance in our medical illustration.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at https://gs.amegroups.com/article/view/10.21037/gs-22-101/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-101/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-101/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Seoul National University Bundang Hospital (IRB No. B-2107-698-104) and informed consent was waived in view of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jung KW, Won YJ, Hong S, et al. Prediction of Cancer Incidence and Mortality in Korea, 2020. Cancer Res Treat 2020;52:351-8. [Crossref] [PubMed]
- Kim JW, Lee JH, Kim TG, et al. Breast reconstruction statistics in Korea from the Big Data Hub of the Health Insurance Review and Assessment Service. Arch Plast Surg 2018;45:441-8. [Crossref] [PubMed]
- Baker BG, Irri R, MacCallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [Crossref] [PubMed]
- Rehnke RD, Groening RM, Van Buskirk ER, et al. Anatomy of the Superficial Fascia System of the Breast: A Comprehensive Theory of Breast Fascial Anatomy. Plast Reconstr Surg 2018;142:1135-44. [Crossref] [PubMed]
- McKinney P, Edelson R, Terrasse A, et al. Chest-wall deformity following soft-tissue expansion for breast reconstruction. Plast Reconstr Surg 1987;80:442-4. [Crossref] [PubMed]
- Sinow JD, Halvorsen RA Jr, Matts JP, et al. Chest-wall deformity after tissue expansion for breast reconstruction. Plast Reconstr Surg 1991;88:998-1004. [Crossref] [PubMed]
- Cherubino M, Scamoni S, Maggiulli F, et al. Breast reconstruction by tissue expansion: What is the integrity of the chest wall? J Plast Reconstr Aesthet Surg 2016;69:e48-54. [Crossref] [PubMed]
- Makiguchi T, Atomura D, Nakamura H, et al. Quantitative assessment and risk factors for chest wall deformity resulting from tissue expansion for breast reconstruction. Breast Cancer 2019;26:446-51. [Crossref] [PubMed]
- Nealon KP, Weitzman RE, Sobti N, et al. Prepectoral Direct-to-Implant Breast Reconstruction: Safety Outcome Endpoints and Delineation of Risk Factors. Plast Reconstr Surg 2020;145:898e-908e. [Crossref] [PubMed]
- Sariguney Y, Ayhan S, Eryilmaz T. Chest wall deformity after tissue expansion. Scand J Plast Reconstr Surg Hand Surg 2008;42:108-9. [Crossref] [PubMed]
- Grodzinsky AJ, Levenston ME, Jin M, et al. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2000;2:691-713. [Crossref] [PubMed]
- Forman JL, Kent RW. The effect of calcification on the structural mechanics of the costal cartilage. Comput Methods Biomech Biomed Engin 2014;17:94-107. [Crossref] [PubMed]
- Holcombe S, Ejima S, Wang S, editors. Calcification of costal cartilage in the adult rib cage. Proceedings of the 2017 International IRCOBI Conference on the Biomechanics of Injury; 2017.
- Whitfield GA, Horan G, Irwin MS, et al. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol 2009;90:141-7. [Crossref] [PubMed]
- Myung Y, Son Y, Nam TH, et al. Objective assessment of flap volume changes and aesthetic results after adjuvant radiation therapy in patients undergoing immediate autologous breast reconstruction. PLoS One 2018;13:e0197615. [Crossref] [PubMed]
- de Bakker E, Rots M, Buncamper ME, et al. The Baker Classification for Capsular Contracture in Breast Implant Surgery Is Unreliable as a Diagnostic Tool. Plast Reconstr Surg 2020;146:956-62. [Crossref] [PubMed]
- Sobti N, Weitzman RE, Nealon KP, et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci Rep 2020;10:1137. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]


