
A case of matrix-producing carcinoma of the breast treated with preoperative chemotherapy
Introduction
Matrix-producing carcinoma (MPC) is a rare tumor accounting for 0.1% of all breast cancers (1). MPC is usually triple-negative breast cancer [TNBC; estrogen receptor (ER)-negative, progesterone receptor (PgR)-negative, and human epidermal growth factor receptor 2 (HER2)-negative] (2). There have been few reports on preoperative chemotherapy for MPC. We report a case of MPC treated with preoperative chemotherapy. We present the following case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-179/rc).
Case presentation
A 47-year-old woman underwent ultrasonography due to feeling a mass in her right breast. Ultrasonography revealed a 3-cm mass in the right upper outer quadrant. A core needle biopsy revealed invasive ductal carcinoma. She was referred to our department for further evaluation and treatment. She had a history of endometriosis and manic-depressive illness.
Clinically, a 3.3-cm elastic, hard, smooth-surfaced mass was palpable in the right upper outer quadrant. There were no skin changes or axillary lymph node swelling.
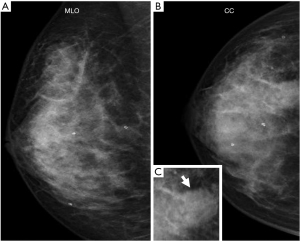
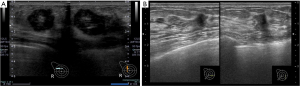
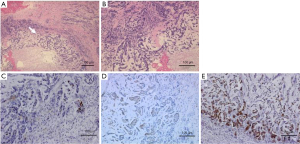
Tumor marker tests revealed carcinoembryonic antigen levels of 1.0 ng/mL (<5.0 ng/mL) and cancer antigen 15-3 of 5.9 U/mL (<27 U/mL), which were within the normal ranges. Mammography revealed a radiopaque lump with a circular shape and a finely serrated edge in the right upper outer quadrant, which was classified as category 4 according to the Breast Imaging Reporting and Data System (Figure 1). The lesion appeared as a circular, hypoechoic, and heterogeneous nodule in the upper outer quadrant of the right breast on ultrasonography. It had a maximum diameter of 2.8 cm (Figure 2A). Another hypoechoic lesion with a maximum diameter of 0.9 cm was observed in the lower inner quadrant of the ipsilateral breast (Figure 2B). Magnetic resonance imaging (MRI) revealed a 3.8 cm tumor in the upper outer quadrant of the right breast. Contrast-enhanced MRI showed a high-intensity lesion with a central low-intensity area (Figure 3A). In the lower inner quadrant of the ipsilateral breast, a 1 cm tumor with irregular margins was found (Figure 3B). Computed tomography (CT) revealed no axillary lymph node metastasis or distant metastasis. Pathological findings revealed a well-defined nodular tumor in the upper outer quadrant of the right breast. Moreover, the tumor cells directly transitioned into a cartilaginous and osseous stromal matrix without an intervening spindle cell. Thus, the patient was diagnosed with MPC (Figure 4A,4B). On immunohistochemistry, the tumor cells tested negative for ER, PgR, and HER2. The Ki67 index was 90%, and the p63 minority was positive (Figure 4C). The S-100 protein and cytokeratin 5/6 were positive (Figure 4D,4E). In the right lower inner quadrant, there was a tumor with stromal elastic fiber. Therefore, the patient was diagnosed with invasive ductal carcinoma. Immunohistochemistry revealed that the tissue of this invasive ductal carcinoma was positive for ER and PgR but negative for HER2. It had a Ki67 index of 7%.
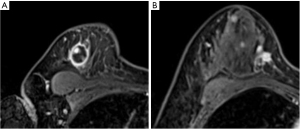
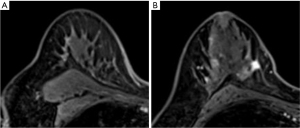
Furthermore, this tumor was staged as cT2N0M0 based on the TNM classification. As a result of the multi-disciplinary team examination, although metaplastic carcinoma has a low sensitivity to chemotherapy, it had a nature of TNBC and neoadjuvant chemotherapy (NAC) would be useful for developing a postoperative treatment strategy. Furthermore, we could discontinue chemotherapy and go forward to surgery in case of tumor progression during NAC administration. We decided to administer NAC with the consent of the patient. EC (epirubicin 90 mg/m2, cyclophosphamide 600 mg/m2) was administered every 3 weeks for a total of 4 courses, followed by 12 courses of weekly paclitaxel (80 mg/m2). After preoperative chemotherapy was completed, the contrast effect in the upper outer quadrant of the right breast mass disappeared on MRI (Figure 5A). The tumor in the lower inner quadrant of the ipsilateral breast exhibited only a 20% reduction at the end of chemotherapy (Figure 5B).
Right skin-sparing mastectomy, sentinel lymph node biopsy, and deep inferior epigastric perforator flap reconstruction were performed under general anesthesia. There was no metastasis to the sentinel lymph nodes. Pathological examination revealed that the cartilage matrix component of the MPC part was hyalinized, and only 0.1 cm of invasive cancer remained at the tumor margin. Regarding invasive ductal carcinoma, the residual tumor diameter of the infiltrated part was 0.7 cm. In both cases, the results of immunostaining for ER, PgR and HER2 in surgical specimen were the same as those of the needle biopsy specimen harvested before NAC. Since a slight residual cancer was found, we considered to administer a postoperative chemotherapy; capecitabine. However, it was not administered due to the patient’s preference. Endocrine therapy with oral tamoxifen was initiated for the invasive ductal carcinoma. Radiation therapy was not performed because there was no metastasis to the lymph nodes. Three years after surgery, no recurrence was observed. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Metaplastic carcinoma is a rare and unique histologic subtype of breast cancer. MPC is categorized as a subtype of metaplastic carcinoma based on the 4th edition of the World Health Organization classification (3). According to the 2016 Annual Breast Cancer Registry by the Japanese Breast Cancer Society, 69 (0.3%) of 25,870 patients with breast cancers were diagnosed with MPC, a rare tumor (4). MPC is an invasive breast carcinoma with a direct transition of carcinoma to the cartilaginous or osseous matrix without an intervening spindle cell component (1). The cartilaginous or osseous matrix components at the tumor center and epithelial carcinoma components at the tumor margin manifest on contrast CT and MRI as ring enhancement, which are important diagnostic findings of MPC (5).
On immunohistochemistry, MPC is usually negative for ER, PgR, and HER2 (2). In addition to the epithelial markers such as keratin and EMA in the carcinoma component, SOX6 (a mesenchymal marker indicating cartilage differentiation), p63 (a myoepithelial cell marker), and S-100 protein (which is positive in chondrocyte-derived tumors) have also been expressed in MPCs (6-8). In our case, both histological and imaging findings were consistent with these typical MPC findings.
Due to the rarity and heterogeneity of MPC, its treatment has not been standardized. Several previous studies have based their treatment plan on the typical TNBC.
In hormone receptor-negative breast cancer, response to preoperative chemotherapy was found to be a prognostic factor (9). Similarly, in patients with metaplastic carcinoma, it is known that the prognosis is good when pathological complete response (pCR) is achieved, regardless of invasive ductal carcinoma (10). Preoperative chemotherapy has a higher clinical value than does postoperative treatment. With the recent emergence of response-guided therapy for breast cancer treatment (11), preoperative chemotherapy is a viable option in patients with TNBC. The pCR rate of typical TNBC from 22% to 38.9% (12,13).
The residual cancer burden (RCB) after preoperative chemotherapy was correlated with prognosis in TNBC. Symmans et al. evaluated the state of residual cancer after preoperative chemotherapy in three stages (RCB-I to III). Their evaluation was based on combining the maximum diameter and length of residual tumor after preoperative chemotherapy, cell density of the invasive cancer in the tumor, proportion of non-invasive cancer in the tumor, number of metastatic lymph nodes, and maximum diameter of lymph node metastasis. The estimated 10-year relapse-free survival rates of the four RCB classes (pathologic complete response, RCB-I, RCB-II, and RCB-III) were 86%, 81%, 55%, and 23% of TNBC (14), respectively.
There have been few reports on preoperative chemotherapy for MPC, and the pCR rate was as low as 0–23% (Table 1) (2,15-18).
Table 1
| Author | Year | N* | pCR | pCR rate |
|---|---|---|---|---|
| Nagao et al. (15) | 2012 | 14 | 0 | 0% |
| Aydiner et al. (16) | 2015 | 8 | 0 | 0% |
| Cimino-Mathews et al. (17) | 2016 | 6 | 1 | 17% |
| Han et al. (18) | 2019 | 17 | 4 | 23% |
| Shimada et al. (2) | 2019 | 5 | 0 | 0% |
*, patients who received preoperative chemotherapy for MPC. MPC, matrix producing carcinoma; pCR, pathological complete response.
Based on previous report, MPC has a worse prognosis than typical breast cancer due to its resistance to chemotherapy, with a 5-year survival rate of approximately 60–86% (19). Recurrences were noted early, within 3 years after surgery (19). In our case, preoperative chemotherapy for MPC was administered to evaluate the patient’s response to chemotherapy and provide optimal treatment. RCB classes of the MPC part corresponded to RCB-I (20), and the patient had a good prognosis. In conclusion, we reported a case of MPC that was successfully treated with preoperative chemotherapy. Since it is rare, a standardized treatment method for MPC has not been established. To investigate the effectiveness of NAC in MPC, analysis of large case series such as national registry data is useful and development of a new targeted therapy to MPC is warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-179/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-179/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-179/coif). AY serves as an unpaid editorial board member of Gland Surgery from April 2019 to March 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Downs-Kelly E, Nayeemuddin KM, Albarracin C, et al. Matrix-producing carcinoma of the breast: an aggressive subtype of metaplastic carcinoma. Am J Surg Pathol 2009;33:534-41. [Crossref] [PubMed]
- Shimada K, Ishikawa T, Yamada A, et al. Matrix-producing Carcinoma as an Aggressive Triple-negative Breast Cancer: Clinicopathological Features and Response to Neoadjuvant Chemotherapy. Anticancer Res 2019;39:3863-9. [Crossref] [PubMed]
- Lakhani SR EI, Schnitt SJ, Tan PH, et al. WHO Classification of Tumours of the Breast. In: World Health Organization classification of tumors. 4th ed. Lyon: International Agency for Research on Cancer, 2012;60-1.
- Kubo M, Kumamaru H, Isozumi U, et al. Annual report of the Japanese Breast Cancer Society registry for 2016. Breast Cancer 2020;27:511-8. [Crossref] [PubMed]
- Koufopoulos N, Kokkali S, Antoniadou F, et al. Matrix-producing Breast Carcinoma: A Rare Subtype of Metaplastic Breast Carcinoma. Cureus 2019;11:e5188. [Crossref] [PubMed]
- Vagia E, Mahalingam D, Cristofanilli M. The Landscape of Targeted Therapies in TNBC. Cancers (Basel) 2020;12:916. [Crossref] [PubMed]
- Shui R, Bi R, Cheng Y, et al. Matrix-producing carcinoma of the breast in the Chinese population: a clinicopathological study of 13 cases. Pathol Int 2011;61:415-22. [Crossref] [PubMed]
- Kusafuka K, Muramatsu K, Kasami M, et al. Cartilaginous features in matrix-producing carcinoma of the breast: four cases report with histochemical and immunohistochemical analysis of matrix molecules. Mod Pathol 2008;21:1282-92. [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- Haque W, Verma V, Schwartz MR, et al. Neoadjuvant Chemotherapy for Metaplastic Breast Cancer: Response Rates, Management, and Outcomes. Clin Breast Cancer 2022;22:e691-9. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275-81. [Crossref] [PubMed]
- Huober J, von Minckwitz G, Denkert C, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010;124:133-40. [Crossref] [PubMed]
- Symmans WF, Wei C, Gould R, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol 2017;35:1049-60. [Crossref] [PubMed]
- Nagao T, Kinoshita T, Hojo T, et al. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast 2012;21:289-95. [Crossref] [PubMed]
- Aydiner A, Sen F, Tambas M, et al. Metaplastic Breast Carcinoma Versus Triple-Negative Breast Cancer: Survival and Response to Treatment. Medicine (Baltimore) 2015;94:e2341. [Crossref] [PubMed]
- Cimino-Mathews A, Verma S, Figueroa-Magalhaes MC, et al. A Clinicopathologic Analysis of 45 Patients With Metaplastic Breast Carcinoma. Am J Clin Pathol 2016;145:365-72. [Crossref] [PubMed]
- Han M, Salamat A, Zhu L, et al. Metaplastic breast carcinoma: a clinical-pathologic study of 97 cases with subset analysis of response to neoadjuvant chemotherapy. Mod Pathol 2019;32:807-16. [Crossref] [PubMed]
- Rakha EA, Tan PH, Shaaban A, et al. Do primary mammary osteosarcoma and chondrosarcoma exist? A review of a large multi-institutional series of malignant matrix-producing breast tumours. Breast 2013;22:13-8. [Crossref] [PubMed]
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22. [Crossref] [PubMed]


