
The prognostic role of surgery and a nomogram to predict the survival of stage IV breast cancer patients
Introduction
The incidence of newly diagnosed primary metastatic breast cancer (pMBC) is between 3% and 25% (1,2). The 5- and 10-year overall survival (OS) rates for women with metastatic breast cancer are only approximately 28% and 13%, respectively (3). The treatment for breast cancer is comprehensive (4). Systemic therapy is the cornerstone of treatment (5); however, controversy remains as to whether the surgical excision of the primary tumor in pMBC patients produces survival benefits (6). Previous evidence indicates that the growth of metastatic lesions (7) might be accelerated by the surgical excision and result in diminished therapeutic effects. Conversely, a number of retrospective (8) studies have suggested that surgical resection is correlated with a survival benefit in pMBC patients (9,10). However, selection bias (11) and other potential confounding factors (12) may have affected these results.
Several prospective trials have shown divergent results (13). For example, the Tata trial and TBCRC 013 (14) found no evidence of a boost in survival benefit due to surgical excision after induction treatment in primary stage IV breast cancer patients (7). Similarly, the ABCSG-28 POSYTIVE trial also found no survival benefit among pMBC patients who underwent surgical excision of the primary tumor followed by systemic therapy (4). Conversely, the MF07-01 trial found a survival benefit among pMBC patients who underwent excision of the primary tumor followed by systemic therapy at a median follow-up period of 40 months (15).
The 5th European School of Oncology-European Society for Medical Oncology international consensus guidelines (16) for breast cancer (version 4.2021), which featured updates to the National Comprehensive Cancer Network guidelines (17), do not provide definite views on surgical therapy for de novo metastatic breast cancer patients. Similarly, at the 17th St. Gallen International Breast Cancer Conference (18), it was noted that due to the many controversies related to the surgical resection of metastatic breast cancer, more randomized data and further research efforts are needed.
Since there is no conclusive conclusion on the efficiency of breast surgery on pMBC patients, we need a prediction model of prognosis, judge the impact of resection of the primary tumor, and identify the metastatic breast cancer patients who would benefit from it. We analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program database, performed 1:1 matched propensity score matching (PSM), constructed a nomogram, performed mutual validation, and divided the stage IV breast cancer patients into a low-risk group and a high-risk group. We present the following article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-330/rc).
Methods
Study population
We analyzed data of pMBC patients from the National Cancer Institute’s SEER Program database between January 1, 2010, and December 31, 2015. Updated annually, SEER currently collects and publishes cancer incidence, and survival data from population-based cancer registries, which routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, the first course of treatment, and follow-up for vital status. The population data used by SEER in calculating cancer rates are obtained periodically from the Census Bureau, and the mortality data reported are provided by the National Center for Health Statistics. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
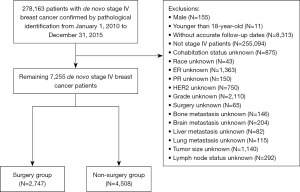
We included 278,163 patients with primary invasive breast cancer confirmed by pathological diagnosis and excluded 255,094 patients with non-metastatic breast cancer. Patients who were male (n=155), who were younger than 18 years at diagnosis (n=11), and who did not have accurate follow-up dates (n=8,313) were excluded from the study. In addition, patients with unknown information on their cohabitation status (n=875), race (n=43), status of estrogen receptor (ER; n=1,363), status of progestogen receptor (PR; n=150), status of human epidermal growth factor receptor-2 (HER2; n=750), tumor grade (n=2,110), surgery (n=65), bone metastasis (n=146), brain metastasis (n=204), liver metastasis (n=82), lung metastasis (n=115), tumor size (n=1,140), and lymph node status (n=292) were also excluded from the study. The follow-up period ran from the date of breast cancer diagnosis to death or December 31, 2015. The inclusion process is illustrated in Figure 1. Ultimately, 4,508 and 2,747 metastatic breast cancer patients were included in the non-surgery and surgery groups, respectively. Patients in the non-surgery group were assigned to the training set, and those in the surgery group were assigned to the validation set.
Demographic, clinicopathological and treatment characteristics
We extracted information on demographic (age at diagnosis, year of diagnosis, race, cohabitation status, education, and income), tumor (stage, size, lymph node status, histotype, grade, ER, PR, and HER2 statuses, and metastasis to the bone, liver, lung, and brain) and treatment (surgery, radiotherapy, and chemotherapy) characteristics and categorized patients into different risk groups based on the median of risk score.
The primary and secondary outcomes were breast cancer-specific survival (BCSS) and OS, respectively. Breast cancer-specific mortality (BCSM; code: 26000) was clarified by the Cause of Death Recode.
Statistical analysis
The characteristics of patients in the surgery group and the non-surgery group were compared by the Student’s t-test or the chi-square test. PSM was applied to establish a 1:1 matched sample comprising pairs of subjects in the surgery group and non-surgery group by the optimal matching algorithm with the “nearest” method and a caliper value of 0.02. A survival analysis was conducted to determine the BCSS and OS of patients in the surgery group and non-surgery group using Kaplan-Meier curves and the log-rank test. Cumulative mortality rates were plotted by considering the competing risks. The mortality rates and hazard ratios of OS and BSCM were calculated to compare patients in the surgery group and non-surgery group.
The potential risk factors that influenced BCSS among patients in the non-surgery group were identified by univariate and multivariate Cox regression models. Covariates with a P value <0.1 in the univariate Cox model were taken into consideration, and those with a P value <0.05 were identified as candidate risk factors in the multivariate model. Based on the scores of the candidate risk factors, a nomogram was constructed to predict BCSS among patients in the non-surgery group at 3 and 5 years after cancer diagnosis. Patients in the non-surgery group were assigned to the training set, and those in the surgery group were assigned to the validation set. Internal validation in the training set and external validation in the validation set were performed to assess the precision of the nomogram using a bootstrap validation method with 1,000 resamples. The concordance index and calibration curves were applied to measure the discrimination of the model. All the patients were further classified into two low- and high-risk groups based on the median of the total score derived from the candidate risk factors in the nomogram. Stratified analyses and tested potential interactions between the risk score groups and surgery were performed using the Wald test.
All the statistical analyses were completed with STATA version 14.1 and various packages (e.g., matchit, tableone, and survival) in R software (version 3.6.1). A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Compared to patients with pMBC in the non-surgery group, those in the surgery group were more likely to be diagnosed in earlier years [2010–2012], be younger at the time of diagnosis, cohabit, and have achieved higher levels of education, but have lower income levels. Additionally, patients in the surgery group had larger tumor sizes, more positive lymph nodes, and better tumor grades, and were more likely to have triple-negative breast cancer (TNBC) but less likely to have metastatic sites in the lung, brain, liver or bone than patients in the non-surgery group. Additionally, patients in the surgery group underwent more radiotherapy and chemotherapy than their counterparts in the non-surgery group (see Table 1).
Table 1
| Variables | All patients | Non-surgery group | Surgery group | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N=7,255 | % | N=4,508 | % | N=2,747 | % | ||||
| Age (years), mean ± SD | 59.5±14.2 | 60.5±14.1 | 57.9±14.2 | <0.001 | |||||
| Age (years) | <0.001 | ||||||||
| 18–39 | 626 | 8.6 | 330 | 7.3 | 296 | 10.8 | |||
| 40–59 | 3,053 | 42.1 | 1,829 | 40.6 | 1,224 | 44.6 | |||
| ≥60 | 3,576 | 49.3 | 2,349 | 52.1 | 1,227 | 44.7 | |||
| Race | 0.64 | ||||||||
| White | 5,423 | 74.7 | 3,360 | 74.5 | 2,063 | 75.1 | |||
| Black | 1,252 | 17.3 | 792 | 17.6 | 460 | 16.7 | |||
| Other | 580 | 8.0 | 356 | 7.9 | 224 | 8.2 | |||
| Cohabitation status | <0.001 | ||||||||
| Non-cohabitation | 3,824 | 52.7 | 2,503 | 55.5 | 1,321 | 48.1 | |||
| Cohabitation | 3,431 | 47.3 | 2,005 | 44.5 | 1,426 | 51.9 | |||
| Education | <0.001 | ||||||||
| Low | 3,385 | 46.7 | 2,186 | 48.5 | 1,199 | 43.6 | |||
| High | 3,870 | 53.3 | 2,322 | 51.5 | 1,548 | 56.4 | |||
| Income | <0.001 | ||||||||
| Low | 3,943 | 54.3 | 2,380 | 52.8 | 1,563 | 56.9 | |||
| High | 3,312 | 45.7 | 2,128 | 47.2 | 1,184 | 43.1 | |||
| Year of diagnosis | <0.001 | ||||||||
| 2010–2012 | 3,361 | 46.3 | 1,878 | 41.7 | 1,483 | 54.0 | |||
| 2013–2015 | 3,894 | 53.7 | 2,630 | 58.3 | 1,264 | 46.0 | |||
| Tumor grade | <0.001 | ||||||||
| Well/moderately differentiated | 2,424 | 53.8 | 1,128 | 41.1 | 2,424 | 53.8 | |||
| Poorly/un-differentiated | 2,084 | 46.2 | 1,619 | 58.9 | 2,084 | 46.2 | |||
| Histotype | 0.24 | ||||||||
| IDC | 5,731 | 79.0 | 3,572 | 79.2 | 2,159 | 78.6 | |||
| ILC | 626 | 8.6 | 399 | 8.9 | 227 | 8.3 | |||
| Others | 898 | 12.4 | 537 | 11.9 | 361 | 13.1 | |||
| Tumor size (cm) | 0.023 | ||||||||
| (0–2] | 1,073 | 14.8 | 707 | 15.7 | 366 | 13.3 | |||
| (2–5] | 3,438 | 47.4 | 2,110 | 46.8 | 1,328 | 48.3 | |||
| >5 | 2,744 | 37.8 | 1,691 | 37.5 | 1,053 | 38.3 | |||
| Lymph node status | <0.001 | ||||||||
| Negative | 1,477 | 20.4 | 1,042 | 23.1 | 435 | 15.8 | |||
| Positive | 5,778 | 79.6 | 3,466 | 76.9 | 2,312 | 84.2 | |||
| ER status | <0.001 | ||||||||
| Positive | 5,382 | 74.2 | 3,428 | 76.0 | 1,954 | 71.1 | |||
| Negative | 1873 | 25.8 | 1,080 | 24.0 | 793 | 28.9 | |||
| PR status | <0.001 | ||||||||
| Positive | 4,365 | 60.2 | 2,791 | 61.9 | 1,574 | 57.3 | |||
| Negative | 2,890 | 39.8 | 1,717 | 38.1 | 1,173 | 42.7 | |||
| HER2 status | 0.79 | ||||||||
| Positive | 1,968 | 27.1 | 1,218 | 27.0 | 750 | 27.3 | |||
| Negative | 5,287 | 72.9 | 3,290 | 73.0 | 1,997 | 72.7 | |||
| Molecular subtype | <0.001 | ||||||||
| HR+/HER2+ | 1,294 | 17.8 | 810 | 18.0 | 484 | 17.6 | |||
| HR−/HER2+ | 674 | 9.3 | 408 | 9.1 | 266 | 9.7 | |||
| HR+/HER2− | 4,210 | 58.0 | 2,695 | 59.8 | 1,515 | 55.2 | |||
| TN | 1,077 | 14.8 | 595 | 13.2 | 482 | 17.5 | |||
| Bone metastasis | <0.001 | ||||||||
| Yes | 4,977 | 68.6 | 3,242 | 71.9 | 1,735 | 63.2 | |||
| No | 2,278 | 31.4 | 1,266 | 28.1 | 1,012 | 36.8 | |||
| Brain metastasis | <0.001 | ||||||||
| Yes | 503 | 6.9 | 389 | 8.6 | 114 | 4.1 | |||
| No | 6,752 | 93.1 | 4,119 | 91.4 | 2,633 | 95.9 | |||
| Liver metastasis | <0.001 | ||||||||
| Yes | 2,007 | 27.7 | 1,386 | 30.7 | 621 | 22.6 | |||
| No | 5,248 | 72.3 | 3,122 | 69.3 | 2,126 | 77.4 | |||
| Lung metastasis | <0.001 | ||||||||
| Yes | 2,380 | 32.8 | 1,634 | 36.2 | 746 | 27.2 | |||
| No | 4,875 | 67.2 | 2,874 | 63.8 | 2,001 | 72.8 | |||
| Chemotherapy | <0.001 | ||||||||
| No/unknown | 2,874 | 39.6 | 2,034 | 45.1 | 840 | 30.6 | |||
| Yes | 4,381 | 60.4 | 2,474 | 54.9 | 1,907 | 69.4 | |||
| Radiotherapy | <0.001 | ||||||||
| No/unknown | 4,679 | 64.5 | 3,189 | 70.7 | 1,490 | 54.2 | |||
| Yes | 2,576 | 35.5 | 1,319 | 29.3 | 1,257 | 45.8 | |||
PSM, propensity score matching; SD, standard deviation; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; TN, triple-negative (HR−HER2−).
Survival and cumulative mortality rates after PSM
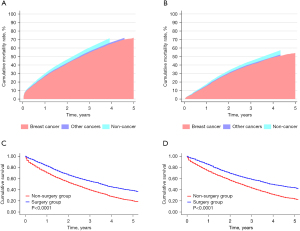
After PSM, the total number of patients in both groups (surgery and non-surgery) was 2,288. The covariates between the two groups were comparable (see Table 2). The median follow-up time of patients in the surgery and non-surgery groups was both 23 months. There are 1,023 breast cancer-specific deaths in the surgery group and 1,313 deaths in the non-surgery group. The cumulative rate of BCSM in the non-surgery group (see Figure 2A) was higher than that in the surgery group (72.10% vs. 54.10%, respectively) (see Figure 2B). Additionally, during the 5-year follow-up period after cancer diagnosis, both the OS (see Figure 2C) and BCSS (see Figure 2D) of the patients in the surgery group were significantly improved compared to those in the non-surgery group.
Table 2
| Variables | Non-surgery group | Surgery group | P value | |||
|---|---|---|---|---|---|---|
| N=2,288 | % | N=2,288 | % | |||
| Age (years), mean ± SD | 59.1±13.7 | 58.8±14.3 | 0.48 | |||
| Age (years) | 0.139 | |||||
| 18–39 | 191 | 8.3 | 226 | 9.9 | ||
| 40–59 | 1,015 | 44.4 | 971 | 42.4 | ||
| ≥60 | 1,082 | 47.3 | 1,091 | 47.7 | ||
| Race | 0.534 | |||||
| White | 1,727 | 75.5 | 1,721 | 75.2 | ||
| Black | 395 | 17.3 | 382 | 16.7 | ||
| Other | 166 | 7.3 | 185 | 8.1 | ||
| Cohabitation status | 0.679 | |||||
| Non-cohabitation | 1,140 | 49.8 | 1,155 | 50.5 | ||
| Cohabitation | 1,148 | 50.2 | 1,133 | 49.5 | ||
| Education | 0.812 | |||||
| Low | 1,004 | 43.9 | 1,013 | 44.3 | ||
| High | 1,284 | 56.1 | 1,275 | 55.7 | ||
| Income | 0.613 | |||||
| Low | 1,294 | 56.6 | 1,276 | 55.8 | ||
| High | 994 | 43.4 | 1,012 | 44.2 | ||
| Year of diagnosis | 0.375 | |||||
| 2010–2012 | 1,167 | 51.0 | 1,136 | 49.7 | ||
| 2013–2015 | 1,121 | 49.0 | 1,152 | 50.3 | ||
| Tumor grade | 0.721 | |||||
| Well/moderately differentiated | 1,026 | 44.8 | 1,013 | 44.3 | ||
| Poorly/un-differentiated | 1,262 | 55.2 | 1,275 | 55.7 | ||
| Histotype | 0.659 | |||||
| IDC | 1,814 | 79.3 | 1,789 | 78.2 | ||
| ILC | 187 | 8.2 | 199 | 8.7 | ||
| Others | 287 | 12.5 | 300 | 13.1 | ||
| Tumor size (cm) | 0.846 | |||||
| (0–2] | 329 | 14.4 | 329 | 14.4 | ||
| (2–5] | 1,077 | 47.1 | 1,095 | 47.9 | ||
| >5 | 882 | 38.5 | 864 | 37.8 | ||
| Lymph node status | 0.36 | |||||
| Negative | 434 | 19.0 | 409 | 17.9 | ||
| Positive | 1,854 | 81.0 | 1,879 | 82.1 | ||
| Molecular subtype | 0.85 | |||||
| HR+/HER2+ | 391 | 17.1 | 408 | 17.8 | ||
| HR−/HER2+ | 227 | 9.9 | 216 | 9.4 | ||
| HR+/HER2− | 1,307 | 57.1 | 1,293 | 56.5 | ||
| TN | 363 | 15.9 | 371 | 16.2 | ||
| Bone metastasis | 1 | |||||
| Yes | 1,512 | 66.1 | 1,511 | 66.0 | ||
| No | 776 | 33.9 | 777 | 34.0 | ||
| Brain metastasis | 0.674 | |||||
| Yes | 103 | 4.5 | 110 | 4.8 | ||
| No | 2,185 | 95.5 | 2,178 | 95.2 | ||
| Liver metastasis | 0.676 | |||||
| Yes | 531 | 23.2 | 544 | 23.8 | ||
| No | 1,757 | 76.8 | 1,744 | 76.2 | ||
| Lung metastasis | 0.625 | |||||
| Yes | 653 | 28.5 | 669 | 29.2 | ||
| No | 1,635 | 71.5 | 1,619 | 70.8 | ||
| Chemotherapy | 0.459 | |||||
| No/unknown | 828 | 36.2 | 803 | 35.1 | ||
| Yes | 1,460 | 63.8 | 1,485 | 64.9 | ||
| Radiotherapy | 0.359 | |||||
| No/unknown | 1,454 | 63.5 | 1,423 | 62.2 | ||
| Yes | 834 | 36.5 | 865 | 37.8 | ||
PSM, propensity score matching; SD, standard deviation; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; HER2, human epidermal growth factor receptor-2; TN, triple-negative (HR−HER2−).
Potential risk factors and prognostic nomogram for BCSS in patients in the non-surgery group after PSM
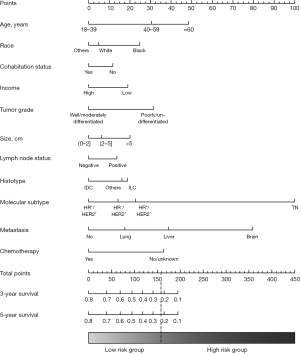
Of the initial 17 variables that were identified to predict the BCSS of patients in the non-surgery group in the univariable Cox regression, 4 variables (i.e., education, year of diagnosis, bone metastasis, and radiotherapy) were excluded. In the multivariable Cox model, we ultimately identified 13 variables (i.e., age, race, cohabitation status, income, tumor grade, histotype, tumor size, lymph node status, molecular subtype, brain metastasis, liver metastasis, lung metastasis, and chemotherapy) as independent predictors of BCSS (see Table 3) and incorporated them into the nomogram (see Figure 3). The scores of the included variables in the nomogram are shown in Table S1. The C statistics for the internal (patients in the non-surgery group) and external (patients in the surgery group) validation of the nomogram were 0.70 [95% confidence interval (CI), 0.69–0.71] and 0.73 (95% CI, 0.72–0.74), respectively. The calibration curves for the nomogram in the training set and validation set also showed high uniformity between the observed outcomes and the nomogram-predicted survival probabilities (see Figure S1).
Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age (years) | <0.001 | <0.001 | |||
| 18–39 | 1 (Reference) | 1 (Reference) | |||
| 40–59 | 1.45 (1.15–1.83) | 0.002 | 1.43 (1.13–1.82) | 0.003 | |
| ≥60 | 1.82 (1.45–2.29) | 0.000 | 1.78 (1.40–2.27) | 0.000 | |
| Race | <0.001 | 0.007 | |||
| White | 1 (Reference) | 1 (Reference) | |||
| Black | 1.44 (1.26–1.65) | 0.000 | 1.25 (1.08–1.44) | 0.002 | |
| Other | 0.93 (0.75–1.16) | 0.510 | 0.96 (0.77–1.19) | 0.686 | |
| Cohabitation status | 0.000 | 0.014 | |||
| Non-cohabitation | 1 (Reference) | 1 (Reference) | |||
| Cohabitation | 0.80 (0.72–0.89) | 0.87 (0.78–0.97) | |||
| Education | 0.091 | 0.188 | |||
| Low | 1 (Reference) | 1 (Reference) | |||
| High | 1.10 (0.99–1.23) | 0.92 (0.81–1.04) | |||
| Income | 0.000 | 0.000 | |||
| Low | 1 (Reference) | 1 (Reference) | |||
| High | 0.76 (0.68–0.85) | 0.77 (0.68–0.88) | |||
| Year of diagnosis | 0.086 | 0.464 | |||
| 2010–2012 | 1 (Reference) | 1 (Reference) | |||
| 2013–2015 | 0.90 (0.80–1.01) | 0.96 (0.85–1.08) | |||
| Tumor grade | <0.001 | <0.001 | |||
| Well/moderately differentiated | 1 (Reference) | 1 (Reference) | |||
| Poorly/un-differentiated | 1.70 (1.52–1.90) | 1.46 (1.29–1.66) | |||
| Histotype | 0.041 | 0.014 | |||
| IDC | 1 (Reference) | ||||
| ILC | 0.96 (0.79–1.18) | 0.717 | 1.26 (1.02–1.56) | 0.029 | |
| Others | 1.22 (1.04–1.43) | 0.013 | 1.20 (1.02–1.41) | 0.027 | |
| Tumor size (cm) | <0.001 | 0.008 | |||
| (0–2] | 1 (Reference) | 1 (Reference) | |||
| (2–5] | 1.01 (0.85–1.19) | 0.915 | 1.07 (0.90–1.26) | 0.462 | |
| >5 | 1.33 (1.13–1.58) | 0.001 | 1.25 (1.05–1.49) | 0.011 | |
| Lymph node status | 0.004 | 0.040 | |||
| Negative | 1 (Reference) | 1 (Reference) | |||
| Positive | 1.23 (1.07–1.42) | 1.17 (1.01–1.36) | |||
| Molecular subtype | <0.001 | <0.001 | |||
| HR+/HER2+ | 1 (Reference) | 1 (Reference) | |||
| HR−/HER2+ | 1.28 (1.01–1.63) | 0.039 | 1.22 (0.96–1.55) | 0.111 | |
| HR+/HER2− | 1.28 (1.09–1.51) | 0.003 | 1.31 (1.09–1.56) | 0.004 | |
| TN | 3.71 (3.07–4.49) | 0.000 | 3.34 (2.75–4.07) | 0.000 | |
| Bone metastasis | <0.001 | 0.143 | |||
| Yes | 1 (Reference) | 1 (Reference) | |||
| No | 1.25 (1.12–1.40) | 0.91 (0.80–1.03) | |||
| Brain metastasis | <0.001 | <0.001 | |||
| Yes | 1 (Reference) | 1 (Reference) | |||
| No | 0.36 (0.29–0.45) | 0.41 (0.32–0.52) | |||
| Liver metastasis | <0.001 | <0.001 | |||
| Yes | 1 (Reference) | 1 (Reference) | |||
| No | 0.75 (0.66–0.85) | 0.62 (0.54–0.71) | |||
| Lung metastasis | <0.001 | <0.001 | |||
| Yes | 1 (Reference) | 1 (Reference) | |||
| No | 0.70 (0.63–0.79) | 0.79 (0.69–0.89) | |||
| Chemotherapy | 0.003 | <0.001 | |||
| No/unknown | 1 (Reference) | 1 (Reference) | |||
| Yes | 0.82 (0.74–0.92) | 0.66 (0.58–0.76) | |||
| Radiotherapy | 0.047 | 0.110 | |||
| No/unknown | 1 (Reference) | 1 (Reference) | |||
| Yes | 1.12 (1.00–1.25) | 1.11 (0.98–1.25) | |||
BCSS, breast cancer-specific survival; PSM, propensity score matching; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; HER2, human epidermal growth factor receptor-2; TN, triple-negative (HR−HER2−); CI, confidence interval.
BCSS and OS of patients in the surgery and non-surgery groups by risk stratification
The risk score derived from patients in the non-surgery group was applied to those in the surgery group. Based on the median risk score, all of the patients were categorized into the following two groups: (I) the low-risk group (a score ≤158, n=3484, 48.0%); and (II) the high-risk group (a score >158, n=3771, 52.0%). As Figure 4 shows, in the low- and high-risk groups, both BCSS and OS were improved among patients in the surgery group. In the low-risk group, patients in the surgery group had lower risks of BCSM (hazard ratio =0.53; 95% CI, 0.47–0.59; P for interaction =0.014) and overall mortality (OM) (hazard ratio =0.52; 95% CI, 0.46–0.58; P for interaction =0.002) than those in the non-surgery group (see Table 4).
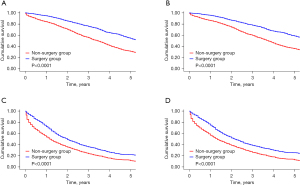
Table 4
| Mortality | Non-surgery group | Surgery group | Hazard ratio (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
| N | MR | N | MR | ||||
| OM | 0.002 | ||||||
| Low-risk group | 975 | 20.3 | 474 | 10.5 | 0.52 (0.46–0.58) | ||
| High-risk group | 1,840 | 53.1 | 917 | 33.1 | 0.63 (0.59–0.69) | ||
| BCSM | 0.014 | ||||||
| Low-risk group | 845 | 17.6 | 420 | 9.3 | 0.53 (0.47–0.59) | ||
| High-risk group | 1,661 | 47.9 | 822 | 29.6 | 0.63 (0.58–0.68) | ||
Risk category defined as low risk (a score ≤158) or high risk (a score >158). OM, overall mortality; BCSM, breast cancer-specific mortality; N, number of deaths; MR, mortality rate per 100 person-years; CI, confidence interval.
Discussion
As stage IV breast cancer is generally uncurable (7), the main goals of de novo metastatic breast cancer treatment are the prolongation of survival, the palliation of symptoms, and the provision of supportive care (19). Controversy remains as to whether the surgical excision of the primary tumor improves the survival of pMBC patients (20). Traditionally, locoregional surgery for patients with distant metastasis is not recommended (21) and has only been used for the control of pain, ulceration, bleeding (22), infection, and fungation.
It is contradictory in terms of the micro mechanism regarding whether locoregional surgery improves the survival of pMBC patients. An analysis of animal models in various cancer experiments indicated that the surgical excision of the primary tumor could accelerate metastatic growth (23,24) by increasing tumor growth factors, fresh surgical dissemination, surgery-induced immunosuppression, and the inflammatory cascade. However, such surgical excision has been shown to lessen the overall tumor burden (25), eliminate the major source of cancer stem cells and tissues that are not sensitive to chemotherapy, and restore immunocompetence (26,27).
Numerous observational studies have shown that locoregional treatment has a favorable effect among women with pMBC (28-30), especially those with limited disease burdens. The reported rate of mortality reduction due to the primary resection ranges from 18–37% (31). However, others have contended that this correlation may be the result of significant biases, and matched analyses have found no survival benefit associated with surgical excision (32). Possible biases include the following: (I) the participants in the studies who agree to the excision of the primary tumor are stage IV breast cancer patients who are healthier and have less underlying disease; (II) the surgery tends to be performed in patients with a lower metastatic disease burden and not in patients with poor projected survival; and (III) the metastatic breast cancer patients who undergo locoregional surgery are more likely to receive more aggressive multimodal therapy.
Some randomized controlled trials, including the Tata trial, TBCRC 013 trial, and ABCSG-28 trial, failed to demonstrate a survival benefit from the resection of the primary tumor in de novo stage IV breast cancer patients, but also found that it causes no survival damage. The Tata trial has been criticized for including patients with considerably advanced disease (74% of the patients had >3 metastatic sites), adopting insufficient systemic therapy strategies, and using outdated treatment sequences. The TBCRC 013 trial was criticized as the follow-up period was not long enough, and the sample size was relatively small, while the ABCSG-28 trial was criticized for failing to obtain negative surgical margins in 20% of patients in the surgery arm, having a small sample size due to poor recruitment, and failing to measure BCSS. In the MF07-01 trial, a statistically significant improvement in survival was observed (median follow-up =40 months) following upfront surgery for stage IV breast cancer patients. However, the patients in the surgery group tended to be younger than 55 years, have ER-positive and HER2-negative tumors, and 47% of the subjects in the Turkish trial had bone-only disease. Thus, these patients may have had early-stage disease rather than advanced disease. The MF07-01 and Tata trials also have a lack of blind method and missing data. Further, they did not measure BCSS or the surgical margins, which led to weak stratification analyses and indeterminate results. Thus, the trials were underpowered and firm conclusions cannot be drawn. In such circumstances, the SEER database is suitable for the study of metastatic breast cancer for its large sample size and relatively long follow-up time.
By analyzing the data from the National Cancer Institute’s SEER Program database, conducting PSM, identifying the potential risk factors that influence the BCSS of patients in the non-surgery group, constructing a nomogram, and conducting mutual validation, we categorized all patients into two groups (low- and high-risk). Compared to patients with metastatic breast cancer in the high-risk group, those in the low-risk group were more likely to be younger at the time of diagnosis, cohabit, have a higher income level, better tumor grades, a histological classification of invasive ductal carcinoma (IDC), smaller tumor sizes, and undergo treatment with more chemotherapy. Conversely, patients in the high-risk group were more likely to be black, have more positive lymph nodes, have TNBC, and have metastatic sites in the brain, liver, and lung.
We found that the excision of the primary tumor distinctly improved the survival of de novo metastatic breast cancer patients in the low-risk group, reducing the OM and BCSM by up to 48% and 47%, respectively. The low-risk group were more likely to obtain benefits from locoregional surgery than the high-risk group (P for interaction of BCSM: 0.014; P for interaction of OM: 0.002). Due to the intrinsic retrospective quality of this study, no decisive conclusion can be drawn at present; however, we found a survival difference between the two groups. Combined with the outcome that the locoregional excision of the primary tumor did not harm the survival outcomes of the pMBC patients, as indicated in some large-scale randomized clinical trials and retrospective studies, we can deduce that the resection of the primary lesion is very likely to be beneficial for patients who meet the characteristics of the low-risk group; however, it is not yet clear whether the locoregional surgery is beneficial for patients who meet the characteristics of the high-risk group. Future multicenter, large-scale prospective studies with long-term follow-up need to be conducted.
Our study had the following limitations: (I) data on patients’ health status, comorbidities, and complications were not collected; (II) detailed information on chemotherapy, radiotherapy, and other systemic treatment strategies was not available in the SEER database, which may be crucial to prognosis; (III) information on the metastasis sites except for bone, lung, liver, and brain, and the number and size of metastases was not available in the SEER database; (IV) information on the timing of surgery of the intact primary tumor, the surgical margin, and patients’ quality of life was not available in the SEER database; (V) the nomograms established in this study have not yet been verified in real-world patients; and (VI) the C statistics for the internal and external validation of the nomogram were 0.70 and 0.73, respectively. Despite the C-index not belonging to high accuracy, the study remains a valuable reference in predicting the survival of stage IV breast cancer patients for its large population.
In conclusion, this study indicated that the surgical removal of the primary tumor might improve the survival of de novo metastatic breast cancer patients in the low-risk group. The established nomogram could provide a reference for clinicians in enabling personalized treatment among advanced breast cancer patients.
Acknowledgments
The authors would like to thank the Surveillance, Epidemiology, and End Results (SEER) Program, which is an authoritative and open-access source for cancer statistics, and which enabled the authors to conduct this reliable population-based study.
Funding: This work was jointly supported by the Key Research and Development Projects of Sichuan Provincial Department of Science and Technology (grant No. 2021YFS0104 to Dr. ZD), Natural Science Foundation of Sichuan Province (grant No. 2022NSFSC0744 to Dr. ZD), Key Projects of Sichuan Provincial Health Commission (grant No. 21PJ042 to Dr. ZD), Incubation Project of West China Hospital of Sichuan University (grant No. 2022HXFH004 to Dr. ZD), and the Full-Time Postdoc Research and Development Foundation of West China Hospital (grant No. 2019HXBH098 to Dr. CW).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-330/rc
Conflicts of Interest: All the authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-330/coif). Dr. ZD reports fundings from the Key Research and Development Projects of Sichuan Provincial Department of Science and Technology (grant No. 2021YFS0104), Natural Science Foundation of Sichuan Province (grant No. 2022NSFSC0744), Key Projects of Sichuan Provincial Health Commission (grant No. 21PJ042), Incubation Project of West China Hospital of Sichuan University (grant No. 2022HXFH004). Dr. CW reports funding from the Full-Time Postdoc Research and Development Foundation of West China Hospital (grant No. 2019HXBH098). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Rossi L, Longhitano C, Kola F, et al. State of art and advances on the treatment of bone metastases from breast cancer: a concise review. Chin Clin Oncol 2020;9:18. [Crossref] [PubMed]
- Eng LG, Dawood S, Sopik V, et al. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res Treat 2016;160:145-52. [Crossref] [PubMed]
- Fitzal F, Bjelic-Radisic V, Knauer M, et al. Impact of Breast Surgery in Primary Metastasized Breast Cancer: Outcomes of the Prospective Randomized Phase III ABCSG-28 POSYTIVE Trial. Ann Surg 2019;269:1163-9. [Crossref] [PubMed]
- Dawood S, Konstantinova M, Perazzo F, et al. Optimizing the management of HER2-negative metastatic breast cancer in the era of PARP inhibitors-proceedings from breast cancer expert group meeting. Chin Clin Oncol 2020;9:61. [Crossref] [PubMed]
- Zheng A, Guo BL, Zhang JG, et al. Clinical information and management status of de novo stage IV breast cancer patients: a Chinese multicenter investigation (CSBrS-002). Chin Med J (Engl) 2021;134:1569-75. [Crossref] [PubMed]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Warschkow R, Güller U, Tarantino I, et al. Improved Survival After Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-adjusted, Population-based SEER Trend Analysis. Ann Surg 2016;263:1188-98. [Crossref] [PubMed]
- Thomas A, Khan SA, Chrischilles EA, et al. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988-2011. JAMA Surg 2016;151:424-31. [Crossref] [PubMed]
- Pons-Tostivint E, Kirova Y, Lusque A, et al. Survival Impact of Locoregional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancers in a Large Multicentric Cohort Study: A Propensity Score-Matched Analysis. Ann Surg Oncol 2019;26:356-65. [Crossref] [PubMed]
- Israel I, Margenthaler JA. Surgical Extirpation of the Primary Tumor in Stage IV Breast Cancer: The Debate Continues. J Am Coll Surg 2021;233:751-2. [Crossref] [PubMed]
- Soran A, Ozmen V, Ozbas S, et al. Primary Surgery with Systemic Therapy in Patients with de Novo Stage IV Breast Cancer: 10-year Follow-up; Protocol MF07-01 Randomized Clinical Trial. J Am Coll Surg 2021;233:742-51.e5. [Crossref] [PubMed]
- Si Y, Yuan P, Hu N, et al. Primary Tumor Surgery for Patients with De Novo Stage IV Breast Cancer can Decrease Local Symptoms and Improve Quality of Life. Ann Surg Oncol 2020;27:1025-33. [Crossref] [PubMed]
- King TA, Lyman JP, Gonen M, et al. Prognostic Impact of 21-Gene Recurrence Score in Patients With Stage IV Breast Cancer: TBCRC 013. J Clin Oncol 2016;34:2359-65. [Crossref] [PubMed]
- Soran A, Ozmen V, Ozbas S, et al. Randomized Trial Comparing Resection of Primary Tumor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann Surg Oncol 2018;25:3141-9. [Crossref] [PubMed]
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. NCCN guidelines® insights: Breast cancer, version 4.2021: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2021;19:484-93. [Crossref] [PubMed]
- Reinert T, de Souza ABA, Sartori GP, et al. Highlights of the 17th St Gallen International Breast Cancer Conference 2021: customising local and systemic therapies. Ecancermedicalscience 2021;15:1236. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Lin Y, Huang K, Zeng Q, et al. Impact of breast surgery on survival of patients with stage IV breast cancer: a SEER population-based propensity score matching analysis. PeerJ 2020;8:e8694. [Crossref] [PubMed]
- Li X, Huang R, Ma L, et al. Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast 2019;45:104-12. [Crossref] [PubMed]
- Arnedos M, Vicier C, Loi S, et al. Precision medicine for metastatic breast cancer--limitations and solutions. Nat Rev Clin Oncol 2015;12:693-704. [Crossref] [PubMed]
- Retsky M, Bonadonna G, Demicheli R, et al. Hypothesis: Induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients. Breast Cancer Res 2004;6:R372-4. [Crossref] [PubMed]
- Al-Sahaf O, Wang JH, Browne TJ, et al. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann Surg 2010;252:1037-43. [Crossref] [PubMed]
- Rashid OM, Nagahashi M, Ramachandran S, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery 2013;153:771-8. [Crossref] [PubMed]
- Norton L, Massagué J. Is cancer a disease of self-seeding? Nat Med 2006;12:875-8. [Crossref] [PubMed]
- Yu Y, Hong H, Wang Y, et al. Clinical Evidence for Locoregional Surgery of the Primary Tumor in Patients with De Novo Stage IV Breast Cancer. Ann Surg Oncol 2021;28:5059-70. [Crossref] [PubMed]
- Lane WO, Thomas SM, Blitzblau RC, et al. Surgical Resection of the Primary Tumor in Women With De Novo Stage IV Breast Cancer: Contemporary Practice Patterns and Survival Analysis. Ann Surg 2019;269:537-44. [Crossref] [PubMed]
- Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 2008;247:732-8. [Crossref] [PubMed]
- Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol 2006;24:2743-9. [Crossref] [PubMed]
- Harris E, Barry M, Kell MR. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol 2013;20:2828-34. [Crossref] [PubMed]
- Teshome M. Role of Operative Management in Stage IV Breast Cancer. Surg Clin North Am 2018;98:859-68. [Crossref] [PubMed]


