
The use of indocyanine green as the only tracer for the identification of the sentinel lymph node in breast cancer: safety and feasibility
Introduction
Axillary staging based on sentinel lymph node biopsy (SLNB) became the gold standard in early-stage breast cancer management and can avoid complete axillary dissection in more than 70% of cases (1-3).
Giuliano et al. (4), in the Z0011 trial (phase 3, 2011) showed that completion axillary dissection is not mandatory in case of positivity of the SLNB in conservative breast surgery. Other ongoing trials (randomized control SENOMAC trial) are evaluating the possibility of omitting complete axillary dissection in case of sentinel lymph node positivity even in case of mastectomy (5).
Therefore, the techniques that allow accurate identification of this lymph node have a remarkable importance, in order to achieve an oncologically correct staging.
A first revolution of the standard technique with methylene blue occurred with the introduction of the radioisotope Technetium-99, first in combination with methylene blue and then alone. With a detection level of up to 96% (6,7) the approach based on preoperative lymphoscintigraphy with Technetium-99 became the gold standard. Some limitations of this procedure are logistical and economical. They include: the need for a department of Nuclear Medicine to obtain the radioisotope and to inject the compound with proper timing; the need for radiation protection; the risk, even if minimal, of the use of a radioactive compound. These are the most important reasons that led the scientific community to search a new tracer, which could overcome the constraints of the radioisotope, while maintaining comparable detection rates of the sentinel lymph node.
Indocyanine green (ICG) is a water-soluble molecule, which is relatively non-toxic and inexpensive. After intravenous injection, it can be rapidly combined with plasma albumin and α-1-lipoprotein, and generate near infrared light penetrating into tissues under the excitation of exotic light with wavelength 750–810 nm. Initially, ICG as a diagnostic drug has been used in clinical practice since the mid-1950s. In 1954, it was approved by the United States Food and Drug Administration for the evaluation of liver reserve function and cardiac circulation function. After that, the unique fluorescence properties of ICG have attracted wide attention. Through the near infrared imaging system equipment, ICG enables form clear fluorescence imaging during surgery, which achieves the visualization of surgical field and real-time tracer drainage of lymphatic and lymph nodes, distinguishes lymphatic tissue from other tissues such as gastric perivascular, fat, pancreas, ureter, etc. And owing to enhanced permeability and retention effect of solid tumors, ICG has the ability to be gathered in tumor tissues, clear display the boundary of the tumors, for the purpose of guiding the complete resection of the tumor.
In the field of oncological surgery, the injection of ICG in the surroundings of the cancer is a method for detecting the sentinel lymph node in tumors of different districts, such as colon, prostate, uterus and oropharynx (8). Its use in breast cancer has been proposed in recent years by the breast surgery community. European Society of Medical Oncology (ESMO) and Japanese Breast Cancer Society (JBCS), define it as the only recommended alternative to the use of radioisotope (9-11).
The use of this tracer is also expanding in Europe: in 2020 in France the FLUOBREAST trial ended (12).
This monocentric observational study proposes ICG as the only tracer for the research and identification of the sentinel lymph node in a high-volume center for breast cancer, evaluating its safety and feasibility.
The unexpected advent of the SARS-CoV-2 (severe acute respiratory syndrome) pandemic has led to a sudden disruption of routine medical care, with a subsequent reorganization of hospital structures and resettlement of therapeutic algorithms. Extraordinary measures have been advocated in order to protect patients and health professionals, and to create a safe path to treat patients (13).
The current healthcare emergency has also become an opportunity for the application of new technologies in clinical practice, as tools to improve patient’s management flow, whilst dealing with limited resources. The use of ICG as a method for identification of the sentinel lymph node fits into this context.
The identification of the sentinel lymph node through the use of the ICG as the only tracer, can be done directly in the operating room, immediately after the induction of general anaesthesia; thus, not requiring patient’s pre-hospitalization, explains why we thought that its preferential use over lymphoscintigraphy could significantly impact preoperative times. Additionally, by avoiding lymphoscintigraphy, our aim was to benefit both the patients (avoiding going another time to the hospital) and the healthcare personnel (decreasing patient-patient and patient-physician interaction).
The aim of the study is to demonstrate the safety and feasibility of the SLNB method using ICG as the only tracer; the primary endpoint is to investigate the sensitivity of this technique compared with Technetium (Tc-99m).
As a secondary endpoint, we evaluated the operative timing and the uptake pathways in relation to the physical characteristics of the patients and the cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-609/rc).
Methods
From March 2020 to February 2021, 184 patients with breast cancer cN0, candidate to SLNB were enrolled at Breast Surgery of Verona.
The inclusion criteria were:
- Patients between 18 and 90 years old;
- cT1-2 breast cancer;
- Axillary lymph nodes negative on ultrasound or MRI investigations, or with negative axillary biopsy (cN0) candidates for SLNB.
The exclusion criteria were:
- Pregnancy or lactation;
- Previous homolateral complete axillary dissection.
All the patients included in the study were informed of the procedure and were asked to sign a specific informed consent. All the procedure of SLNB was performed using Verdye®—Diagnostic Green Gmbh (ICG) as only tracer.
ICG is a vital fluorescent dye with a molecular weight of 776 Da, which turns out a dark green coloration in ambient light and fluorescence spectrum in the infrared wavelength (700 nm to 1 mm).
For intraoperative and transcutaneous observation of ICG fluorescence, was used the photodynamic system NOVADAQ SPY Elite system (Stryker®).
This system uses a Laser diode at 806 nm to stimulate up to 18.5×13.5 cm2 surface and a high-resolution camera (1,024×768) for recording the fluorescent signal. The photodynamic system causes the excitation of the fluorescent tracer that emits a light signal in the infrared spectrum: this signal is then captured by the high-resolution camera, creating a real time image on monitor.
This procedure is carried out in the operating room with the lights off, to improve the identification of the light signal. The depth of observation allowed by the system is about 3 cm, even on the skin.
Medical equipment allows four different display modes (Figure 1): white light mode, color mode with fluorescence overlay (OVERLAY), color segmental fluorescence (CSF) mode for qualitative quantification of the concentration of the ICG tracer, fluorescence SPY mode.
In the operating room, during induction of anaesthesia and before the preparation of the surgical field, surgeons injected a total of 1.0 mL of Verdye® (25 mg/mL reconstituted with 10 mL of NaCl 0.9%) dividing the amount in 4 shots of 0.25 mL in the peri-areolar site, approximately at 3, 6, 9, 12 o’clock. After the injection, the breast was gently massaged to facilitate lymphatic uptake of the dye.
At the end of this phase, time t0 was recorded.
After preparation the surgical field, through the use of the fluorescence camera (SPY Portable Handheld Imagining System, NOVADAQ Stryker®), the team identified and followed the tracer pathways (Figure 2). The incision was then made at the point where the fluorescent signal visually ended in the axillary region.
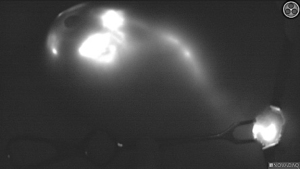
The time t1 and the number of pathways the tracer was reported on the procedure form.
Once the locus of absorption maximum of the tracer was identified, the fluorescent tissues were removed, and time t2 was recorded. The fluorescent tissue from the axillary region, was checked ex vivo with colour segmental fluorescence mode (CSF mode) to identify the sentinel lymph node and accessory-sentinel lymph nodes.
The axillary tissues were then evaluated with the SPY Camera to confirm the complete removal of the fluorescent lymph node tissue. Even palpable or macroscopically suspicious lymph nodes, although not fluorescent, were removed and sent as accessory lymph nodes.
The following data was recorded during the surgical procedure: time of tracer injection (t0), axillary incision time (t1) and time of extraction of the sentinel lymph node (t2). We also reported the characteristics inherent the mapping of the lymphatic drainage, such as the number of lymphatic pathways originating from the areola, the number of spy foci, the number of lymph nodes sent as a sentinel and the number of foci sent as accessory nodes.
We reported the failure of the procedure in one of the following situations: absence or overlay of illumination by the tracer in the axillary region, absence of identification of lymphatic drainage pathways or absence of identification of the sentinel lymph node.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was waived because the procedures performed in this study involving human participants is already validated.
Statistical analysis
IBM® SPSS Statistics, version 25, and Microsoft Excel 365® (16.43 version, 20110804) were used for Student’s t-test statistical analysis.
Results
A total of 184 cases were enrolled in the study (2 males, 182 females).
Patients’ median age was 65.8 years (range, 27–90 years), with a median body mass index (BMI) of 25.09 kg/m2 (range, 14.13–41.78 kg/m2). The most common histological subtype was ductal invasive carcinoma (n=109; 59.2%), followed by lobular invasive carcinoma (n=42; 22.8%).
Ninety patients had comorbidities such as arterial hypertension, diabetes mellitus type II, dyslipidemia, as shown in Table 1; 30 patients were smokers.
Table 1
| Variable | Value |
|---|---|
| Gender | |
| Male | 2 |
| Female | 182 |
| Age, years | 65.8 (27–90) |
| 0–49 | 26 |
| 50–69 | 74 |
| >70 | 84 |
| BMI, kg/m2 | 25.09 (14.13–41.78) |
| <18 | 3 |
| 18–<25 | 94 |
| 25–<30 | 64 |
| ≥30 | 23 |
| Comorbidity (90/184) | |
| Arterial hypertension | 59/90 |
| Diabetes | 16/90 |
| Dyslipidemia | 30/90 |
| Active smoker | 30 |
Data are presented as mean (range) or numbers. BMI, body mass index.
Some patients had previously undergone medical, radiotherapy or surgical therapies; in detail, as shown in Table 2, 12 patients underwent neoadjuvant chemotherapy (NACT), 20 performed external radiotherapy (of whom 19 for previous breast cancer and 1 for previous Hodgkin lymphoma), 47 undergone breast surgery and 27 undergone axillary surgery (18 subjected to SLNB and 9 patients subjected to axillary sampling) (Table 2).
Table 2
| Variable | N |
|---|---|
| NACT (n=12) | |
| Anthracyclines + taxanes scheme | 12 |
| Previous radiotherapy (n=20) | |
| Conservative treatment of breast cancer | 19 |
| Hodgkin lymphoma | 1 |
| Previous breast surgery (n=47) | |
| Tumorectomy | 35 |
| Simple mastectomies | 3 |
| Simple mastectomies with reconstruction | 8 |
| Bilateral breast augmentation | 1 |
| Previous axillary surgery (n=27) | |
| SLNB | 18 |
| Axillary sampling | 9 |
NACT, neoadjuvant chemotherapy; SLNB, sentinel lymph node biopsy.
The sentinel lymph node was detected and excised in 181 out of 184 cases (98.4%). The average number of total lymph nodes (TLNs) removed was 2.9 for single patient, the average number of lymph nodes classified intra-operatively as sentinel was 1.56. These results are consistent with the recommendations in the literature about the minimal and maximal number of excised lymph nodes in breast surgery (14,15).
Lymph node metastases were found in 31 patients within SLNs (16.8%), whereas no micro- or macro-metastases in accessory SLN were reported, suggesting that this technique might be highly selective. So, the anatomopathological concordance between the sentinel lymph node and metastatic localizations was 100%: in all patients with metastatic lymph nodes, they always appeared also in the sentinel one. No lymphatic station “jump” occurred in the process of lymphatic spread by cancer cells (“skipping metastases”).
The average t0 and t1 were 11 minutes and 03 seconds and 14 minutes and 7 seconds respectively. Median sentinel lymph node incision time (SLNIT; t1-t0) and sentinel lymph node excision time (SLNET; t2-t1) were 11 minutes and 14 minutes, respectively (range, 11–16 minutes; range, 12–18 minutes). So, the median length of procedure was 25 minutes (range, 11–40 minutes). The average number of lymph node uptake pathways was 1.59, with a range between 0 and 4.
Following periareolar ICG injection, at least one fluorescent pathway leading to the axilla could be highlighted in 95.5% patients, 52.3% patients had a single pathway, while 34.1% had 2 pathways and 9.1% had 3 separate pathways to the axilla.
In addition, we analysed the possible existence of predictive factors for the time of lymph node removal (SLNET) and for the number of uptake pathways.
None of the patients (even those allergic to iodium) presented immediate reactions even in the absence of premedication (16,17).
No skin tattoos residual from the tracer were found.
Discussion
The SARS-CoV-2 pandemic has completely disrupted the routine of standard medical treatments, allowing the rapid evolution of new technological approaches. The use of ICG as a method of identifying the sentinel lymph node fits into this context. From March 2020 to February 2021, breast cancer patients treated at the UOC of Breast Surgery of the Azienda Ospedaliera Universitaria Integrata (AOUI) of Verona, performed the SLNB with the ICG as only tracer.
A number of studies reported an SLN detection rate using fluorescence-guided imaging techniques ranging from 96% to 99%, with an average of retrieved SLN ranging from 2.1 to 3.5 (18-23).
In our cohort, SLNs were found in 98.3% of patients, with an average number of SLNs retrieved of 1.5, while the average number of TLNs was 2.9, as guidelines recommend for a standard SLNB procedure.
In the present cohort, none of the identified micro- or macro-metastases was found within accessory SLN, suggesting that this technique might be highly selective, further reducing the risk of morbidity that comes with additionally lymph node removal during the procedure.
ICG procedure has proven efficacy for patients who have previously undergone surgery (breast and/or axillary) or radiotherapy in the homolateral thoracic region.
Most studies show a length of procedure to a median of 26 minutes (range, 15–50 minutes), with a non-significant P if performed under local anesthesia or general anesthesia (19). These data are comparable to the results of the present study (24).
In addition, we analysed the possible existence of predictive factors for the time of lymph node extraction and for the uptake pathways displayed on a monitor.
Dimension and breast weight (within the subgroup of mastectomies), are possible predictors for the extraction times. To our knowledge, this is the first study to correlate these parameters to SLN operative times. The rationale behind the association of breast dimensional parameters and SLN excision time is unclear. This might be related to a more represented adipose tissue that may alter the outflow of the tracer, and/or to the limited sensitivity of the camera for signals deeper than 3 cm. A possible solution for this could be to implement an intraoperative boost injection of 1 mL of the same ICG solution in close proximity to the tumor or along the fluorescent ICG pathway to enhance SLN’s tracer concentration. Further studies are needed to clarify our findings on breast’s dimensional parameters, as they could represent an obstacle for the surgeon when using near infrared fluorescence-guided surgery for SLN identification.
A statistically significant difference (P=0.0077) was then found in the extraction time for patients with previous ipsilateral breast surgery. This difference could be justified by the lymphatic drainage of the breast as a result of the prior surgery.
The average number of lymph node uptake pathways was 1.59, with a range between 0 and 4.
Following periareolar ICG injection, at least one fluorescent pathway leading to the axilla could be highlighted in 95.5% patients, 52.3% patients had a single pathway, while 34.1% had 2 pathways and 9.1% had 3 separate pathways to the axilla.
To our knowledge, the present study is the first one to relate clinical and pathological features to the number of fluorescent pathways leading to the axilla.
In our analysis, a statistically significant difference was highlighted in the rate of G3 disease (16.0% vs. 52.6%; P=0.013), estrogen receptor (ER) negative disease (4.0% vs. 31.6%; P=0.013), triple negative disease (4.0% vs. 26.3%; P=0.033) and median number of fluorescent foci within the axilla (1 vs. 2; P=0.041) between patients with more than one pathway leading to the axilla (Group B, more than 2 pathways) and patients with a single or no pathway (Group A, 0–1 pathway).
We believe that this association could be explained by the fact that aggressive tumors might have a greater lymphatic vascularization produced by a more intense angiogenesis, hence a greater number of draining pathways in the armpit, compared to lower grade tumors. Alternatively, it can be speculated that aggressive tumors might have a higher rate of lymphatic infiltration, which might impair lymphatic flow to the point that multiple drainage pathways are needed to reach the axilla. Based on these findings, it could be reasonable to assume that patients with more than one draining pathway could benefit from an SLN frozen section.
Although these results are promising and could pave the way for interesting speculations, further studies are warranted to clarify the associations of biological parameters with lymphatic drainage pathway patterns.
Patients in Group B underwent mastectomy more frequently than patients in Group A (78.9% vs. 44.0%; P=0.02). Additionally, the median number of fluorescent foci within the axilla was higher in Group B than in Group A (2 vs. 1; P=0.041). There was a trend for median TLNs to be higher in Group B than in Group A (P=0.068).
No differences in terms of SLNIT, and SLNET could be identified between the two groups (all P>0.05).
Finally, we analysed the influence of multifocality and/or multicentricity of the lesion on the number of uptake pathways, finding a significant difference from unifocal lesions. Among the hypotheses aimed at explaining this finding, we include the lack of the “ab extrinseco” compression effect of the lymphatic system by the tumor mass, and the absence of intravascular lymphatic metastases that could cause the occlusion of the lymphatic vessel, and therefore a reduction of the uptake pathways.
The results of the present study, combined with the most recent experiences described in literature, confirm the feasibility, efficacy and safety of the ICG technique, which can be suggested as a single tracer for SLNB. This is particularly valid in hospitals where there is no nuclear medicine service or in which a double hospital access of the patient is not logistically possible, an undeniable advantage especially in pandemic era (24). Additionally, this technique seems to be cost effective, especially where the system and the tracer used are shared with general surgeons, urologists and gynaecologists (25-27). In our university hospital, both tracer and camera are used only for study purposes. The ICG tracer is used by general surgeons for the intra-operative study of intestinal anastomoses, by plastic surgeons for the vitality of the areola-nipple complex, and even by urologists to identify the sentinel lymph node in penile tumors. The main limiting factor to a routine use of this technique seems to be the lack of indication to the extravasal use of the tracer.
Conclusions
This work seems to suggest that the use of ICG tracer only in the SLNB procedure in patients with cN0 breast cancer, might be a safe, effective, and sensitive technique. Cost analyses are necessary in order to effectively determine whether the routine use of this tracer results in a reduction in material and health personnel costs compared to the lymphoscintigraphic technique.
This method may in the future completely replace the use of radiocolloid as the main tracer for SLNB procedures, reducing costs and management time that the standard procedure normally entails.
Acknowledgments
All authors thank Stryker® corporation for the assistance about NOVADAQ SPY Elite system, Prof. G. P. Pollini for the skills provided throughout the clinical study, and E. Filippi for the administrative and statistical support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-609/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-609/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-609/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-609/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was waived because the procedures performed in this study involving human participants is already validated. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer 2017;17:379. [Crossref] [PubMed]
- Derossis AM, Fey J, Yeung H, et al. A trend analysis of the relative value of blue dye and isotope localization in 2,000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg 2001;193:473-78. [Crossref] [PubMed]
- Bines S, Kopkash K, Ali A, et al. The use of radioisotope combined with isosulfan Blue dye is not superior to radioisotope alone for the identification of sentinel lymph nodes in patients with breast cancer. Surgery 2008;144:606-9; discussion 609-10. [Crossref] [PubMed]
- Xiong L, Gazyakan E, Yang W, et al. Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol 2014;40:843-9. [Crossref] [PubMed]
- Sugie T, Ikeda T, Kawaguchi A, et al. Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol 2017;22:11-7. [Crossref] [PubMed]
- Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi7-23. [Crossref] [PubMed]
- Jinno H, Inokuchi M, Ito T, et al. The Japanese Breast Cancer Society clinical practice guideline for surgical treatment of breast cancer, 2015 edition. Breast Cancer 2016;23:367-77. [Crossref] [PubMed]
- Ngô C, Sharifzadehgan S, Lecurieux-Lafayette C, et al. Indocyanine green for sentinel lymph node detection in early breast cancer: Prospective evaluation of detection rate and toxicity-The FLUOBREAST trial. Breast J 2020;26:2357-63. [Crossref] [PubMed]
- Smidt ML, Janssen CM, Barendregt WB, et al. Sentinel lymph node biopsy performed under local anesthesia is feasible. Am J Surg 2004;187:684-7. [Crossref] [PubMed]
- Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. [Crossref] [PubMed]
- Diagnostic green. Product information (PIL). Available online: https://diagnosticgreen.com/row/product-information/
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. [Crossref] [PubMed]
- Krohne TU, Allam JP, Novak N, et al. "Iodine allergy": A medical myth with risks for the ophthalmological patient. Ophthalmologe 2016;113:1023-8. [Crossref] [PubMed]
- Rubinchik-Stern M, Shmuel M, Bar J, et al. Maternal-fetal transfer of indocyanine green across the perfused human placenta. Reprod Toxicol 2016;62:100-5. [Crossref] [PubMed]
- Papadia A, Mohr S, Imboden S, et al. Laparoscopic Indocyanine Green Sentinel Lymph Node Mapping in Pregnant Cervical Cancer Patients. J Minim Invasive Gynecol 2016;23:270-3. [Crossref] [PubMed]
- Cox CE, Dupont E, Whitehead GF, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J 2002;8:88-91. [Crossref] [PubMed]
- Derossis AM, Fey JV, Cody HS 3rd, et al. Obesity influences outcome of sentinel lymph node biopsy in early-stage breast cancer. J Am Coll Surg 2003;197:896-901. [Crossref] [PubMed]
- Takei H, Suemasu K, Kurosumi M, et al. Added value of the presence of blue nodes or hot nodes in sentinel lymph node biopsy of breast cancer. Breast Cancer 2006;13:179-85. [Crossref] [PubMed]
- Pellini F, Di Filippo G, Mirandola S, et al. Effects of Lean Thinking and Emerging Technologies on Breast Cancer Patients' Therapeutic Process During COVID-19 Pandemic: A Case-Control Matched Study. Front Surg 2021;8:582980. [Crossref] [PubMed]
- Somashekhar SP, Kumar CR, Ashwin KR, et al. Can Low-cost Indo Cyanine Green Florescence Technique for Sentinel Lymph Node Biopsy Replace Dual Dye (Radio-colloid and Blue Dye) Technique in Early Breast Cancer: A Prospective Two-arm Comparative Study. Clin Breast Cancer 2020;20:e576-83. [Crossref] [PubMed]
- Fan Z, Zong J, Lau WY, et al. Indocyanine green and its nanosynthetic particles for the diagnosis and treatment of hepatocellular carcinoma. Am J Transl Res 2020;12:2344-52. [PubMed]
- Egloff-Juras C, Bezdetnaya L, Dolivet G, et al. NIR fluorescence-guided tumor surgery: new strategies for the use of indocyanine green. Int J Nanomedicine 2019;14:7823-38. [Crossref] [PubMed]
- Hu Y, Fu T, Zhang Z, et al. Does application of indocyanine green fluorescence imaging enhance clinical outcomes in liver resection? A meta-analysis. Photodiagnosis Photodyn Ther 2021;36:102554. [Crossref] [PubMed]



