
Papillary thyroid cancer with suspicious uterine cervix metastasis: a case report and literature review
Introduction
Papillary thyroid cancer (PTC) is the most common type of thyroid malignancy and accounts for the recent major increase in the incidence observed worldwide (1,2). Locoregional lymphatic spread is frequent in PTC and is reported in approximately 30–90% of cases (3). However, PTC is well-known for its relatively indolent disease course and favorable prognosis, with a 5-year survival rate of more than 95% and a 10-year survival rate of up to 90% (4).
Distant metastasis (DM) of PTC is rare, with a 1–9% incidence at the initial diagnosis or during follow-up (5,6), and the most common site of DM is the lung followed by the bone, and rare metastases are found in the brain, breast, kidney, liver, and skin (7). DM of PTC is associated with substantial decrease in the survival rate, with a reported 10-year disease-specific survival rate of 50–75% (8).
An aggressive treatment strategy is required in the management of PTC with DM. However, rare metastatic sites of PTC are neither easily predicted nor detected in the clinical setting, leading to their oversight during treatment. Accurate clinical staging at initial diagnosis and during follow-up is essential.
In this report, we present a rare case in which a suspicious uterine cervix metastasis was detected in a patient with PTC during follow-up after total thyroidectomy. Empirical evidence of persistently elevated serum thyroglobulin (Tg) levels and a suspicious finding in the whole-body scan (WBS) led to the clinical suspicion of uterine cervix metastasis from PTC. Surgical resection in the form of total hysterectomy confirmed the pathology of chronic cervicitis and the absence of malignancy. However, decreased serum Tg levels with negative image findings after surgery provided insight into the patient’s current disease status and guidance to future treatment strategy. We present the following case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-210/rc).
Case presentation
Patient
A 38-year-old woman was referred to our hospital for an incidentally discovered thyroid nodule located in the isthmus, which was diagnosed as PTC by fine needle aspiration biopsy (Bethesda category VI). She had no noticeable symptoms and no past medical history except for surgical excision of breast fibroadenoma. She had neither thyroid disease nor irradiation exposure and had no family history of thyroid cancer.
Physical examination of the neck revealed a hard, ill-defined, movable nodule in the left isthmus measuring approximately 0.6 cm in diameter. There was no cervical lymphadenopathy. Laboratory serum tests including complete blood count, electrolytes, and thyroid function were normal. Ultrasound (US) of the neck showed a solid, hypoechoic, irregular nodule with microcalcification in the left lower isthmus. The lesion measured 0.64 cm in diameter at the longest point on the longitudinal view, and anterior capsular invasion (r/o T3a stage) was suspected. An enlarged extrathyroidal nodule with a round shape, 0.6 cm in maximum diameter, was identified in the left lower posterior portion, suspicious of a reactive lymph node (LN) or parathyroid lesion.
A cervical magnetic resonance imaging (MRI) scan with gadolinium contrast revealed that an approximately 0.5 cm enhancing mass at the left isthmus was seen with clear lining of the trachea, and the sternothyroid muscles seemed intact. No abnormal LN enhancement was found. None of the MRI findings suggested pulmonary or bone metastases.
Thyroid surgery
Left hemithyroidectomy with isthmusectomy under general anesthesia was recommended for the patient. A hard, irregular nodule was detected with intact thyroid capsules. After thyroidectomy, elective ipsilateral central compartment neck dissection (CCND) was conducted. The procedure included level 6 prelaryngeal, pretracheal, and paratracheal LNs. During pretracheal LN dissection, three enlarged, dark-colored LNs were detected and resected. Harvested LNs were sent for frozen analysis and were reported as three metastatic LNs with a maximum diameter of 0.6 cm. When we performed left paratracheal LN dissection, an enlarged LN was discovered, and frozen analyses revealed one 0.6 cm metastatic LN. As all resected LNs were identified as metastases, we proceeded to perform completion thyroidectomy. The patient was informed of the multiple clinically apparent central LN metastases and subsequent completion thyroidectomy. The patient was administered 100 µg of levothyroxine from postoperative day 1 and had an uneventful postoperative recovery without any complications.
Pathology
Gross examination of the total thyroidectomy with CCND specimen showed an ill-defined whitish infiltrative mass in the isthmus, measuring 0.7 cm × 0.5 cm × 0.3 cm (Figure 1A). In hematoxylin and eosin (H&E)-stained slides, papillary microcarcinoma with a classic papillary structure was identified (Figure 1B). There was focal microscopic extrathyroidal extension without resection margin involvement (Figure 1C). Multiple psammoma bodies were scattered in the background thyroid parenchymal tissue combined with chronic lymphocytic thyroiditis. Immunohistochemical staining of D2-40 (podoplanin) identified lymphatic vessel invasion around the tumor (Figure 1D), which was not clearly observed in the H&E slides. Blood vessel invasion was absent. Molecular studies showed the presence of a mutation in B-Raf proto-oncogene, serine/threonine kinase (BRAF) [Ch 7q34, exon 15, V600E point mutation (c.1799T>A)]; telomerase reverse transcriptase (TERT) promotor mutation was negative (Ch 5p15.33, TERT C228T, C250T point mutation negative).
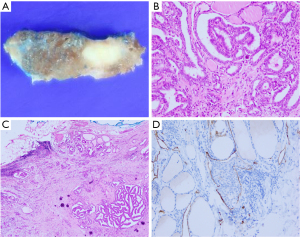
All four dissected central LNs were confirmed as metastatic carcinomas without extranodal extension, and the largest metastatic deposit size was measuring up to 0.6 cm in diameter. According to the 8th edition of the American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) classification system, the pathological stage was determined as T1aN1aM0, stage I.
Radioactive iodine (RAI) treatments after surgery—the first therapy
Adjuvant RAI ablation treatment was indicated for the patient due to the presence of microscopic extrathyroidal extension, lymphovascular invasion of the tumor, and N1 disease involving LNs with 0.6 cm in largest dimension, which were the intermediate risk factors for disease recurrence according to the 2015 American Thyroid Association (ATA) risk stratification system. After 1 month of cessation of levothyroxine medication and exogenous stimulation with recombinant human thyroid stimulating hormone (TSH), she received RAI ablation treatment by administration of 150 mCi I-131 (postoperative 2 months). A WBS following therapeutic RAI ablation showed residual uptake in the thyroid bed alone.
Six-month follow-up neck US and chest computed tomography (CT) showed no recurrence. Two LNs (measuring 0.4 and 0.3 cm), which were seen in the left lower neck, level 6, were noted for close monitoring. The serum Tg level was 1.3 ng/mL, with a TSH level of 1.11 µIU/mL (postoperative 8 months). The presence of measurable on-thyroxine serum Tg levels and the equivocal US findings indicated an incomplete response of RAI treatment. Additional RAI treatment was therefore determined.
RAI treatments after surgery—the second therapy
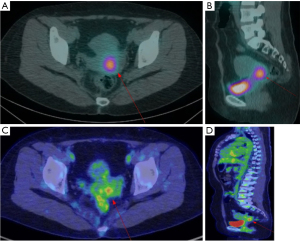
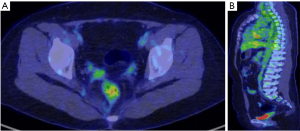
Second RAI ablation therapy was administered at a dose of 150 mCi after 2 months (postoperative 10 months). A WBS following therapeutic RAI ablation showed a focal uptake in the uterine cervix (Figure 2A,2B, arrows). Abdominopelvic CT and MRI showed no visible mass or abnormal signal intensity lesion in the cervix. An approximately 3.5 cm-sized septated thin-walled cyst in the left ovary was seen with contrast enhancement in the septum and wall. Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) was performed, showing focal FDG uptake in the uterine cervix with an increased maximum standardized uptake value (SUVmax) of 4.1 (Figure 2C,2D, arrows). No other abnormal uptake was observed. The serum Tg level was 4.5 ng/mL, with a TSH level of 1.12 µIU/mL (postoperative 11 months).
Discussion with the gynecology department for further treatment
The patient was admitted to the gynecologic oncology unit. She had a gynecologic history of frequent vaginitis after cesarean section 8 years ago, but no abnormalities were observed in the gynecologic examination. Her serum carcinoembryonic antigen and cancer antigen 125 levels were 3.8 ng/mL and 16.3 U/mL, respectively, which were within normal range. The human papillomavirus Pap test was negative. Pelvis MRI showed no visible mass or abnormal signal intensity lesion in the uterine cervix, and a 3.5 cm benign-looking septate cyst in the left ovary was noted. Cystography showed no demonstrable vesicocervical fistula.
Since there was no definite region of malignancy in the uterine cervix, the possibility of hidden malignancies in other regions should have also been considered. Instead of other options, additional RAI treatment with a follow-up WBS was first recommended. If the follow-up WBS showed persistent uptake in the uterine cervix, hysterectomy was proposed as one treatment option for the patient.
RAI treatments after surgery—the third therapy
A third round of RAI ablation therapy was administered at a dose of 180 mCi (postoperative 15 months). The same focal uptake in the uterine cervix was observed on the posttherapeutic WBS. No uptake in other parts of the genitourinary tract was found. In addition, the new appearance of diffuse radioiodine uptake in the gallbladder led us to suspect inflammatory change.
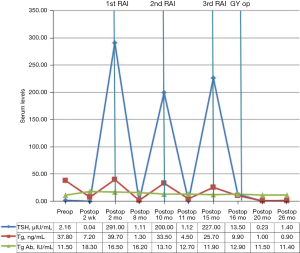
Follow-up neck US and chest CT showed no evidence of recurrence in the neck and the lung. Abdominopelvic CT revealed a 2 cm benign-looking cystic lesion in the left adnexa and a suspicious 1.9 cm intramural uterine myoma. Fundal adenomyomatosis of the gallbladder was noticed. The serum Tg level was 9.9 ng/mL, with a TSH level of 13.5 µIU/mL (postoperative 16 months). Serial serum Tg and TSH levels of the patient are described in Figure 3.
Gynecologic surgery and pathology
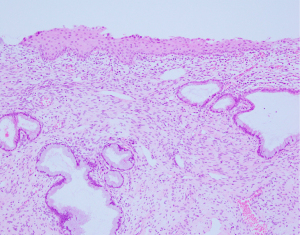
With no future plans for pregnancy and having a significant concern about the disease, the patient instantly decided to undergo further hysterectomy. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy was performed, as scheduled (postoperative 17 months). There were no suspicious findings on gross examination of the uterine cervix; the uterine cervix was entirely embedded through a total of 19 blocks, but there was no evidence of metastatic lesions. Chronic cervicitis with squamous metaplasia, adenomyosis, and leiomyoma were diagnosed in the uterine specimen. No hidden malignancy was observed (Figure 4). The patient had an uneventful postoperative recovery.
After 3 months, follow-up abdominopelvic CT showed persistent fundal adenomyomatosis of the gallbladder and no abnormalities suggestive of DM. Her serum Tg level was reduced to 1.0 ng/mL, with a TSH level of 0.23 µIU/mL (postoperative 20 months) (Figure 5).
The follow-up studies
Six months after the second surgery, the patient was in a good physical condition with no posttherapeutic complications and was eager to go through follow-up investigations to assess her disease status. The follow-up studies have shown a further decrease in the serum level of Tg to 0.9 ng/mL, with a TSH level of 1.4 µIU/mL (Figure 3). Follow-up neck US showed no evidence of recurrence, and no abnormal uptake was seen in the 18F-FDG PET/CT (postoperative 26 months) (Figure 5). A benign cystic lesion in the right adnexa with no uptake was observed. A left ovarian cyst was not definitely seen. The patient was relieved with the results.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PTC metastasis to the female genital tract is a rare event. Few cases have been reported including the involvement of the uterine myometrium, uterine corpus, and ovaries (9-11). Metastasis to the uterine cervix is extremely unusual with no publication in the literature at this time. Occasional cervical metastases have been found in extragenital organ cancers, such as colorectal, stomach, and breast cancers (12).
With a significant negative impact on disease-specific survival and prognosis, DM of PTC should be considered a “possible occurrence at any time” which needs meticulous detection and aggressive treatment. This case had several “intermediate” risk factors for disease recurrence according to the 2015 ATA risk stratification system: minor extrathyroidal extension, lymphovascular invasion of the tumor, and N1 disease involving LNs (4 out of 4) with 0.6 cm in largest dimension. BRAF V600E mutation is associated with aggressive features and poor prognosis (13). Adjuvant RAI ablation treatment was indicated for the patient.
Serum Tg levels represent tumor burden and the capacity of the tumor to synthesize and secrete Tg. This marker is used to detect the recurrence of thyroid cancer after total thyroidectomy with or without RAI treatment (14). Patients with PTC who are considered clinically free of tumor have serum Tg levels <1 ng/mL with suppressed TSH (on-thyroxine).
In this case, elevated on- and off-thyroxine serum Tg levels indicated an incomplete biochemical response that led to a second and a third RAI ablation therapy (13). Moreover, posttherapeutic WBS and 18F-FDG PET/CT showed a preliminary finding of possible uterine cervix metastasis from PTC. After the third RAI ablation therapy, lasting uptake in the uterine cervix was seen, and serum Tg was persistently elevated. After hysterectomy, negative imaging findings and decreased serum Tg levels (≤1 ng/mL) were observed.
The diagnostic accuracy and pitfalls of imaging modalities should be carefully considered. 131I is the leading diagnostic and therapeutic agent for metastatic PTC. The combined results of posttherapeutic WBS and serum Tg testing can lead to the recognition of iodine avidity in the metastatic lesions, which is important in determining further treatment (15). The value of 18F-FDG PET/CT in PTC, which is known as an effective agent in detecting metastatic PTC lesions, especially in 131I WBS-negative, Tg-positive patients after RAI ablation, has also been demonstrated (16).
Regarding 18F-FDG uptake in the uterine cervix and its surgical pathology in our patient, the possibility of false positives should have been considered: from a gynecologic perspective, 18F-FDG uptake can originate from the physiologic renal excretion of FDG into the ureters and the urinary bladder, and increased FDG activity can occur in benign conditions such as uterine fibroids, pelvic inflammatory disease, and benign cystic lesions (17). Since vesicocervical fistula was not observed in this case, the inflammation in the cervix should have been considered together with PTC metastasis. Other study findings indicated that primary malignancy of the uterine cervix was less likely.
Although the patient had a history of frequent vaginitis after C-section delivery, she did not currently have vaginitis or other inflammatory changes. No specific threshold of SUVmax has been demonstrated to discriminate malignancy and inflammation of the cervix. One study reported a cut-off level of 4.0 as a higher probability of non-avidity to RAI in 18F-FDG-positive metastatic differentiated thyroid cancer (15). Our patient’s SUVmax of 18F-FDG uptake in the cervix was 4.1, which was considered clinically significant.
Another possible explanation of elevated Tg levels with uptake in imaging studies is the presence of struma ovarii. There have been several cases of struma ovarii with similar clinical findings (18,19). Our patient also had ovarian cysts, which were noted on CT and MRI. However, no abnormal uptake in the ovaries was observed on WBS and 18F-FDG PET/CT. Furthermore, decreased serum Tg levels and negative imaging findings after hysterectomy would imply that the ovarian cysts were benign and far from being a form of struma ovarii.
Even though the final pathology did not confirm the uterine cervix metastasis, we should consider the possibility of hidden malignancies in the uterine specimen. One study reported a hidden malignancy in asymptomatic uterine leiomyoma that was confirmed as PTC metastasis after surgical removal (20). In our case, we scrutinized the uterine specimen including the leiomyoma, but malignant cells were not identified. However, pathological examinations can be limited and cannot entirely exclude the microscopic metastatic spread to the other portions of the uterus. Since serum Tg levels were further decreased after hysterectomy, we postulate that further RAI ablation therapy with surgical resection was reasonable and not excessive.
Herein, we report a case of PTC with suspicious metachronous uterine cervix involvement. We acknowledge that cervix metastases of PTC are extremely rare, and the final pathology did not confirm metastasis from PTC. This case raises a discussion of differential diagnostic limitations in the setting of clinical and pathological investigations of PTC. Even though hysterectomy may be perceived as excessive treatment, the process leading to the conclusion of additional surgery was rational. Persistently elevated serum Tg levels with clear uptake in the cervix after repetitive high-dose RAI ablation following total thyroidectomy gave rise to reasonable suspicion of DM from PTC. Even with incomplete assurance of DM, our treatment strategies have resulted in the patient’s decreased serum Tg levels, together with negative imaging findings. Based on the relevant rationales, through multidisciplinary discussion, this patient can ultimately obtain a better prognosis. Understanding the pitfalls of the imaging modalities and continuous efforts to overcome the limitations of the diagnostic process are crucial for future treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-210/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-210/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Morris LG, Sikora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013;23:885-91. [Crossref] [PubMed]
- Sakorafas GH, Koureas A, Mpampali I, et al. Patterns of Lymph Node Metastasis in Differentiated Thyroid Cancer; Clinical Implications with Particular Emphasis on the Emerging Role of Compartment-Oriented Lymph Node Dissection. Oncol Res Treat 2019;42:143-7. [Crossref] [PubMed]
- Jeon MJ, Kim WG, Kim TH, et al. Disease-Specific Mortality of Differentiated Thyroid Cancer Patients in Korea: A Multicenter Cohort Study. Endocrinol Metab (Seoul) 2017;32:434-41. [Crossref] [PubMed]
- Kim H, Kim HI, Kim SW, et al. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol Metab (Seoul) 2018;33:287-95. [Crossref] [PubMed]
- Toraih EA, Hussein MH, Zerfaoui M, et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers (Basel) 2021;13:1625. [Crossref] [PubMed]
- Yang J, Ma Y, Gong Y, et al. Multiple Simultaneous Rare Distant Metastases as the Initial Presentation of Papillary Thyroid Carcinoma: A Case Report. Front Endocrinol (Lausanne) 2019;10:759. [Crossref] [PubMed]
- Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg 2010;251:114-9. [Crossref] [PubMed]
- Newman C, O’Leary M, Quill D, et al. Papillary Thyroid Cancer Presenting as a Uterine Metastasis. Journal Of Endocrinology And Metabolism 2019;9:113-6. [Crossref]
- Wang JH, Yu J, Ning CP, et al. Papillary thyroid carcinoma with massive metastasis in the uterine corpus: a case report. BMC Cancer 2013;13:551. [Crossref] [PubMed]
- Corrado G, Pomati G, Russo A, et al. Ovarian metastasis from thyroid carcinoma: a case report and literature review. Diagn Pathol 2014;9:193. [Crossref] [PubMed]
- Pérez-Montiel D, Serrano-Olvera A, Salazar LC, et al. Adenocarcinoma metastatic to the uterine cervix: a case series. J Obstet Gynaecol Res 2012;38:541-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Foppiani L, Sola S, Cabria M, et al. Unstimulated Serum Thyroglobulin Levels after Thyroidectomy and Radioiodine Therapy for Intermediate-Risk Thyroid Cancer Are Not Always a Reliable Marker of Lymph Node Recurrence: Case Report and a Lesson for Clinicians. Case Rep Endocrinol 2020;2020:8827503. [Crossref] [PubMed]
- Liu M, Cheng L, Jin Y, et al. Predicting 131I-avidity of metastases from differentiated thyroid cancer using 18F-FDG PET/CT in postoperative patients with elevated thyroglobulin. Sci Rep 2018;8:4352. [Crossref] [PubMed]
- Miller ME, Chen Q, Elashoff D, et al. Positron emission tomography and positron emission tomography-CT evaluation for recurrent papillary thyroid carcinoma: meta-analysis and literature review. Head Neck 2011;33:562-5. [Crossref] [PubMed]
- Lakhani A, Khan SR, Bharwani N, et al. FDG PET/CT Pitfalls in Gynecologic and Genitourinary Oncologic Imaging. Radiographics 2017;37:577-94. [Crossref] [PubMed]
- Oikonomou C, Spathari N, Doumoulaki S, et al. Recurrent Struma Ovarii Presented with High Levels of Thyroglobulin. Case Rep Surg 2021;2021:8868095. [Crossref] [PubMed]
- Ghander C, Lussato D, Conte Devolx B, et al. Incidental diagnosis of struma ovarii after thyroidectomy for thyroid cancer: functional imaging studies and follow-up. Gynecol Oncol 2006;102:378-80. [Crossref] [PubMed]
- Bertrand AS, Iannessi A, Peyrottes I, et al. Myoma Hot Spot: Tumor-to-Tumor Metastasis of Thyroid Origin into Uterine Leiomyoma. Eur Thyroid J 2019;8:273-7. [Crossref] [PubMed]


