
The optimal extent of lymph node dissection in N1b papillary thyroid microcarcinoma based on clinicopathological factors and preoperative ultrasonography
Introduction
Papillary thyroid microcarcinoma (PTMC) is a papillary thyroid carcinoma with a maximum tumor diameter ≤1.0 cm. The Surveilance, Epidemiology, and End Results data showed that in the United States the incidence rate of PTMC increased from 3.39 per 100,000 person-years from 1983 to 1985 to 13.02 per 100,000 person-years from 2010 to 2013, and the average annual change rate was 9.3% (1). Generally, most PTMCs have good clinical results, but some show aggressive behavior, such as extrathyroidal extension (ETE), lateral lymph node metastasis (LLNM), and even distant metastasis at initial diagnosis, which results in a poor prognosis (2). Regional lymph node (LN) metastasis is an independent risk factor for recurrence/persistence in PTMC patients (3,4). The overall recurrence rate of PTMC is 5.45%, and LLNM is an independent factor for recurrence in PTMC patients (5). Involvement on several levels of the neck in N1b PTC patients significantly affects overall recurrence-free survival (RFS) and lateral neck RFS rates (6).
The 2015 American Thyroid Association (ATA) consensus recommended therapeutic lateral neck dissection for N1b papillary thyroid carcinoma (7). The main operative methods include selective neck dissection (SND) and modified radical neck dissection (MRND) (8). The extent of MRND is levels II–V with preservation of the sternocleidomastoid muscle, internal jugular vein, and spinal accessory nerve. SND refers to dissection of the lateral LNs, sparing ≥1 of the lateral neck levels. SND may be accompanied by a high local recurrence rate (9,10), and MRND can result in a certain degree of shoulder dysfunction and sensory changes (6,11), caused by dissection, traction, or transection of the nerve, which impairs the patient’s quality of life (12). The main goal of the clinical management of PTMC is to prevent disease recurrence, reduce patient deaths, and minimize treatment-related adverse events. Achieving the best balance between treatment benefits and complications depends on accurate preoperative evaluation and standardized surgical approach.
Ultrasonography (USG) is the most commonly used imaging technique for evaluating the cervical LNs. Various USG features help predict cervical LN malignancies, such as round shape, microcalcification, hyperechogenicity, cystic appearance, and peripheral vascularity. Preoperative USG has been shown to have high sensitivity and specificity for detecting LLNM in papillary thyroid carcinoma patients (13), so the preoperative USG features of LNs, including size and hyperechogenicity, may be valuable in predicting recurrence of N1b papillary thyroid carcinoma (14).
Therefore, the purpose of this study was to identify the factors associated with the presence of multilevel LLNM in N1b PTMC patients and to discuss the extent of lymphadenectomy needed. In this study, we evaluated the following clinical concerns: (I) patterns and clinical characteristics of LLNM in N1b PTMC; (II) predictive factors of lateral LLNM in N1b PTMC; and (III) the diagnostic value of preoperative USG for detecting metastatic LNs at different neck levels, which may help physicians determine the optimal initial surgical procedure. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-284/rc).
Methods
Patient selection
We conducted a retrospective study of N1b PTMC patients who simultaneously underwent total thyroidectomy, bilateral central LN dissection (CLND), and ipsilateral therapeutic lateral LN dissection (LLND) between January 2019 and June 2021 at Tianjin Medical University Cancer Institute and Hospital. All patients underwent preoperative neck USG, fiberoptic laryngoscopy, and enhanced computed tomography scanning. Suspicious lateral LNs were reported on a “per level” basis and were compared with the postoperative diagnosis of LN metastasis. Therapeutic LLND was performed in patients who underwent fine needle aspiration biopsy plus washout thyroglobulin level or fast-frozen pathology. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Medical Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital (No. bc2022100).Individual consent for this retrospective analysis was waived.
Surgical strategy
Total thyroidectomy and CLND were performed first. CLND consists of the removal of the LNs and fibroadipose tissue between the common carotid arteries laterally from the hyoid bone superiorly to the innominate artery inferiorly, including the prelaryngeal, pretracheal, and paratracheal LNs and the anterior superior mediastinal LNs along the innominate artery (15). SND or MRND was selected based on the neck level of preoperative USG. SND was routinely performed, and the minimum scope of dissection was levels II–IV. Preoperative USG that indicated metastasis of level V or simultaneous 3-level metastasis was an indication for MRND.
Study methods
Firstly, we analyzed the metastic pattern of N1b PTMC patients. Secondly, the patients were divided into two groups: 1-level and multilevel LLNM. The following histopathological factors were assessed: age, sex, primary tumor size, multiplicity, bilaterality, capsule invasion, Hashimoto’s thyroiditis (HT), and pathological subtype. Multiplicity was defined as ≥2 lesions of PTMC in one lobe, regardless of bilaterality. The number of metastatic and harvested LNs in each level was recorded as indicated by the surgeon during the operation. Extranodal extensions (ENEs) of the metastatic LNs were also recorded. The clinicopathological differences between the two groups were recorded and compared.Lastly, to investigate the accuracy of preoperative USG in assessing per level and multilevel metastasis of N1b PTMC.
Statistical analysis
Statistical analysis was performed with SPSS 25.0 software. The chi-squared test or Fisher’s exact test was used for categorical variables, and the independent two-sample t-test was used to compare continuous variables. Multivariate logistic regression analysis was performed on variables with a P value <0.05 in the univariate analysis to assess independent associations of multilevel LLNM. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs), and statistical significance was defined as P<0.05.
Results
Patients’ characteristics
A total of 182 patients with N1b PTMC were enrolled (Table 1), with proportionally more female than male patients. The mean age was 42.1±9.9 years and only 15 patients (8.2%) were older than 55 years of age. The mean tumor size was 7.1 mm, and 48 patients (26.4%) had tumors with a diameter ≤5 mm. There were 72 (39.6%) classical variants, 76 (41.8%) follicular variants, and 34 (18.7%) other variants. Multiplicity, bilaterality, Hashimoto’s thyroiditis, capsule invasion, and ENE were detected in 94 (51.6%), 93 (51.1%), 54 (29.7%), 171 (94.0%) and 134 (73.6%) patients, respectively. The mean central LN metastasis (CLNM) was 4.73±4.46 and the mean central LN ratio was 0.38±0.29. Skip metastasis, defined as LLNM without CLNM, occurred in 28 patients (15.4%). SND was performed in 123 patients (67.6%), and MRND was performed in 59 patients (32.4%).
Table 1
| Variable | Values |
|---|---|
| Age (years), n (%) | |
| Mean ± SD | 42.1±9.9 |
| <55 | 167 (91.8) |
| ≥55 | 15 (8.2) |
| Sex, n (%) | |
| Male | 61 (33.5) |
| Female | 121 (66.5) |
| Tumor size (mm), n (%) | |
| Mean ± SD | 7.1±2.5 |
| ≤5 | 48 (26.4) |
| >5 | 134 (73.6) |
| Multifocality, n (%) | 94 (51.6) |
| Bilaterality, n (%) | 93 (51.1) |
| HT, n (%) | 54 (29.7) |
| Capsule invasion, n (%) | 171 (94.0) |
| Pathological subtype, n (%) | |
| CPTC | 72 (39.6) |
| FV-PTV | 76 (41.8) |
| Variants† | 34 (18.7) |
| CLNM (mean ± SD) | 4.73±4.46 |
| Bilateral CLNM, n (%) | 90 (49.5) |
| CLNR (mean ± SD) | 0.38±0.29 |
| LNM (mean ± SD) | 10.36±7.11 |
| Skip metastasis, n (%) | 28 (15.4) |
| ENE, n (%) | 134 (73.6) |
| Lateral neck dissection, n (%) | |
| SND | 123 (67.6) |
| MRND | 59 (32.4) |
| 2015 ATA recurrence | |
| Risk groups, n (%) | |
| Intermediate-risk | 154 (84.6) |
| High-risk | 28 (15.4) |
†, tall cell, diffuse sclerosing, solid, Warthin-like, clear cell. PTMC, papillary thyroid microcarcinoma; SD, standard deviation; HT, Hashimoto’s thyroiditis; CPTC, classical PTC; FV-PTC, follicular variant, papillary thyroid; CLNM, central lymph node metastasis; CLNR, central lymph node ratio; LNM, lymph node metastasis; ENE, extranodal extension; SND, selective neck dissection; MRND, modified radical neck dissection; ATA, American Thyroid Association.
Patterns of multilevel LLNM
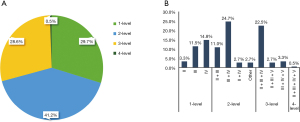
Of the 182 patients with LLNM, level III metastasis was the most common (139/182; 76.4%), followed by level IV (129/182; 70.9%), level II (79/182; 43.4%), and level V (16/59; 27.1%) metastasis. A total of 54 patients (29.7%) had 1-level metastasis, and 128 patients (70.3%) had multilevel metastasis. Levels 1–4 metastasis were detected in 54 (29.7%), 75 (41.2%), 52 (28.6%), and 1 (0.5%) patients, respectively (Figure 1A). The most common pattern was 2-level metastasis (41.2%). The most common metastatic distributions were IV (14.8%) in 1-level metastasis, III + IV (24.7%) in 2-level metastasis, and II + III + IV (22.5%) in 3-level metastasis. Level V metastasis was commonly involved in 3-level (6.0%) and 4-level metastasis (0.5%) (Figure 1B).
Risk factors for multilevel LLNM in N1b PTMC patients
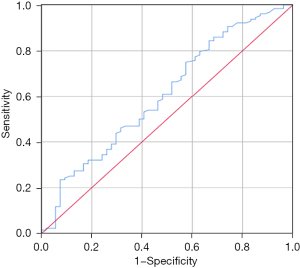
The patients were divided into two groups: 1-level and multilevel LLNM. The clinicopathological differences between the two groups are shown in Table 2. CLNM (3.67±4.54 vs. 5.17±4.36, P=0.037) and central lymph node ratio (CLNR) (0.26±0.24 vs. 0.44±0.27, P=0.001) were significantly lower in patients with 1-level LLNM compared with those with multilevel metastasis. Univariate analysis revealed that capsule invasion, CLNM, CLNR, bilateral CLNM, upper pole location, and ENE were associated with multilevel LLNM, but age, sex, multiplicity, bilaterality, pathological subtype, microcalcification, and HT were not significantly associated. Patients with 1-level LLNM had lower thyroid-stimulating hormone (TSH) levels than those with multilevel LLNM (2.13±1.43 vs. 2.60±1,65, P=0.073). The predictive value of TSH to discriminate between 1-level and multilevel LLNMwas determined by ROC analysis, and the area under the curve (AUC) was 0.607 (95% CI: 0.515–0.698) (P=0.047) (Figure 2). A cut-off value of 1.545 m IU/L for TSH had a sensitivity of 75.0% and a specificity of 42.6% to detect multilevel LLNM. Therefore, we grouped TSH ≤1.5 and >1.5 mIU/L in all models for LLNM.
Table 2
| Variable | 1-level (n=54) | Multilevel (n=128) | χ2/t | P value |
|---|---|---|---|---|
| Age (years), n (%) | ||||
| <55 | 50 (92.6) | 117 (91.4) | 0.071 | 0.790 |
| ≥55 | 4 (7.4) | 11 (8.6) | ||
| Sex, n (%) | ||||
| Male | 18 (33.3) | 43 (33.6) | 0.001 | 0.973 |
| Female | 36 (66.7) | 85 (66.4) | ||
| Tumor size (mm), n (%) | ||||
| ≤5 | 19 (35.2) | 29 (22.7) | 3.070 | 0.080 |
| >5 | 35 (64.8) | 99 (77.3) | ||
| Multifocality, n (%) | ||||
| Absent | 24 (44.4) | 64 (50.0) | 0.469 | 0.493 |
| Present | 30 (55.6) | 64 (50.0) | ||
| Bilaterality, n (%) | ||||
| Absent | 26 (48.1) | 63 (49.2) | 0.017 | 0.895 |
| Present | 28 (51.9) | 65 (50.8) | ||
| Pathological subtype, n (%) | ||||
| CPTC | 19 (35.2) | 53 (41.4) | 1.586 | 0.450 |
| FV-PTV | 22 (40.7) | 54 (42.2) | ||
| Variants† | 13 (24.1) | 21 (16.4) | ||
| Capsule invasion, n (%) | ||||
| Absent | 8 (14.8) | 3 (2.3) | 10.401 | 0.001 |
| Present | 46 (85.2) | 125 (97.7) | ||
| CLNM (mean ± SD) | 3.67±4.54 | 5.17±4.36 | 2.101 | 0.037 |
| CLNR (mean ± SD) | 0.26±0.24 | 0.44±0.27 | 4.001 | 0.001 |
| Bilateral CLNM, n (%) | ||||
| Absent | 35 (64.8) | 57 (44.5) | 6.251 | 0.012 |
| Present | 19 (35.2) | 71 (55.5) | ||
| Microcalcification, n (%) | ||||
| Absent | 14 (25.9) | 22 (17.2) | 1.828 | 0.176 |
| Present | 40 (74.1) | 106 (82.8) | ||
| HT, n (%) | ||||
| Absent | 39 (72.2) | 93 (72.7) | 0.004 | 0.952 |
| Present | 15 (27.8) | 35 (27.3) | ||
| TSH (μIU/mL), n (%) | ||||
| Mean ± SD | 2.13±1.43 | 2.60±1.65 | 1.803 | 0.073 |
| ≤1.5 | 21 (38.9) | 29 (22.7) | 5.022 | 0.025 |
| >1.5 | 33 (61.1) | 99 (77.3) | ||
| Upper pole location, n (%) | ||||
| Absent | 24 (44.4) | 37 (28.9) | 4.115 | 0.043 |
| Present | 30 (55.6) | 91 (71.1) | ||
| ENE, n (%) | ||||
| Absent | 23 (42.6) | 25 (19.5) | 10.402 | 0.001 |
| Present | 31 (57.4) | 103 (80.5) | ||
†, tall cell, diffuse sclerosing, solid, Warthin-like, clear cell. PTMC, papillary thyroid microcarcinoma; CPTC, classical PTC; FV-PTC, follicular variant, papillary thyroid carcinoma: CLNM, central lymph node metastasis; SD, standard deviation; CLNR, central lymph node ratio; HT, Hashimoto’s thyroiditis; TSH, thyroid-stimulating hormone; ENE, extranodal extension.
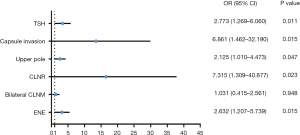
In the multivariate analysis, capsule invasion (OR =6.861, 95% CI: 1.462–32.190, P=0.015), upper pole location (OR =2.125, 95% CI: 1.010–4.473, P=0.047), CLNR (OR =7.315, 95% CI: 1.309–40.877, P=0.023), TSH level (OR =2.773, 95% CI: 1.269–6.060, P=0.011], and ENE (OR =2.632, 95% CI: 1.207–5.739, P=0.015) were independent risk factors for multilevel LLNM (Figure 3).
Diagnostic value of preoperative USG in predicting LLNM in neck levels II–V
A comparison of preoperative USG findings with postoperative pathologic findings is summarized in Table 3. The sensitivity of USG in identifying metastatic lLNs in levels II–V was 62.9%, 83.7%, 80.1%, and 75.0%, respectively, and the specificity was 61.9%, 40.7%, 61.3%, and 78.4%. The sensitivity and specificity of USG for identifying multilevel metastases were 67.2% and 64.8%, respectively.
Table 3
| Neck level | Ultrasonography, n (%) | Histopathology, n (%) | Sensitivity, % | Specificity, % | |||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | ||||
| II | 147 (80.8) | 35 (19.2) | 104 (57.1) | 78 (42.9) | 62.9 | 61.9 | |
| III | 59 (32.4) | 123 (67.6) | 44 (24.2) | 138 (75.8) | 83.7 | 40.7 | |
| IV | 31 (17.0) | 151 (83.0) | 49 (26.9) | 133 (73.1) | 80.1 | 61.3 | |
| V | 51 (86.4) | 8 (13.6) | 42 (71.2) | 17 (28.8) | 75.0 | 78.4 | |
| Multilevel | 128 (70.3) | 54 (29.7) | 77 (42.3) | 105 (57.7) | 67.2 | 64.8 | |
LLNM, lateral lymph node metastasis.
Discussion
This study found that 2-level metastasis was the most common pattern in PTMC and that capsule invasion, upper pole location, CLNR, TSH level, and ENE were independent risk factors for multilevel LLNM. Furthermore, the TSH level could differentiate 1-level from multilevel LLNM with acceptable sensitivity and specificity, and preoperative USG was found to have excellent diagnostic accuracy in evaluating LLNM of primary PTMC.
PTMC generally presents an indolent disease course with a 15-year disease-specific survival rate of 99% and a recurrence rate of 5% (16). However, some PTMCs show aggressive behavior, such as ETE and LLNM at initial diagnosis. In the present series, 72.1% of patients had multilevel LLNM. According to the 2015 ATA Risk Stratification System, 154 cases in our series were classified as intermediate-risk and 28 were classified as high-risk recurrence. Therefore, the decision on a reasonable extent of LN dissection in N1b PTMC should consider the balance between functional protection and oncologic safety.
The pattern of LLNM in PTMC has been investigated previously. In one study the overall frequency of multilevel LLNM was 50.7% (9), whereas we found as overall incidence of 72.1% in patients with N1b PTMC, which may be due to the different extent of SND. In the report by Wang et al., the SND group comprised patients with excision of the lateral neck LNs, sparing ≥1 of the lateral neck levels (9). In the present study, the scope of SND was levels II–IV. Other studies (6,10) have reported that the rate of multilevel LLNM after MRND in patients with N1b PTC was 73.6–74.5%, which is consistent with our results. Simultaneous levels III + IV and II + III + IV metastasis were the most frequently involved in lateral 2- and 3-level metastasis, which was consistent with the anatomic pathways of lymphatic spread in the thyroid region (17). Therefore, comprehensive dissection of levels II–IV may be necessary in patients with N1b PTMC; even if 1- or 2-level metastasis is found, the minimum range of SND should be levels II–IV.
It is still controversial whether level V should be routinely dissected, considering the potential morbidity associated with injury to the spinal accessory nerve (6,11,12). Bhattacharyya reviewed the data of 2,097 patients with papillary thyroid carcinoma and found no statistically significant difference in recurrence or death rates between patients who underwent SND and MRND for nodal metastasis (18). Therefore, several studies have argued against routine level V dissection in patients with N1b PTMC because of the relatively low incidence of level V metastasis and recurrence (19-21). However, level V metastasis, if found simultaneously with 1- or 2-level metastasis, may have a negative impact on locoregional recurrence rate (6). Therefore, it is very important to accurately evaluate the cervical LN level involvement and preoperative USG has shown high sensitivity and specificity for detecting cervical LN metastasis in patients with papillary thyroid carcinoma (13,22). However, previous studies did not clarify the accuracy of USG in the diagnosis of LLNM at different neck levels, which may be helpful in determining the necessity and extent of neck dissection in PTMC. Kang et al. reported that USG has low sensitivity (43.4%) but high specificity (84.0%) in evaluating level V LN metastasis (20). In the present study, the sensitivity of USG in identifying metastatic LNs at level V was 75.0%, and the specificity was 78.4%, but this result may be due to the different inclusion criteria. In their study, routine MRND was performed in all patients, whereas in our study, MRND was performed only when preoperative USG indicated regional V metastasis or simultaneous 3-level metastasis. Other studies have also shown that simultaneous multilevel metastasis is an independent predictor of level V metastasis (9,19,23), which supports our principle of cleaning level V.
Factors predictive of multilevel LLNM were investigated to determine the indications for extensive LND in patients with N1b PTMC. We found that the independent predictors of multilevel metastasis included capsule invasion, upper pole location, CLNR, TSH >1.5 mIU/L, and ENE. Kim et al. found that ETE and bilateral CLNM were independent risk factors for multilevel LLNM in N1b papillary thyroid carcinoma (6). In addition, the incidence of LLNM increased significantly with an increase in CLNM (17). In our study, CLNM in patients with multilevel LLNM was significantly higher than in patients with 1-level metastasis (5.17±4.36 vs. 3.67±4.54, P<0.001). Our study showed that bilateral CLNM was a risk factor for multilevel LLNM, but not an independent risk factor. Our study also showed that CLNR was associated with simultaneous multilevel metastasis, which is similar to the findings reported by Wang et al. (9) The upper pole location was significantly associated with LLNM, as in previous reports (24-26). Tumor cells originating from the upper pole of the thyroid may be transported to the lateral LNs along the superior thyroid artery (27). Our results also showed that tumor location in the upper pole was an independent risk factor for multilevel LLNM. ENE not only increased the risk of LLNM, but was also significantly associated with distant metastasis and disease persistence/recurrence (28,29). We observed that ENE was an independent risk factor and patients with ENE had a 2.63-fold (95% CI: 1.207–5.739) higher risk of multilevel LLNM than those without ENE.
TSH plays a key role in the development of clinical PTMC, and is considered as a risk predictor for tumor progression in PTMC patients (30). Tam et al. reported that the serum TSH level was also associated with increased tumor diameter, bilateral tumors, capsular invasion, and LN metastasis (31). A meta-analysis of 56 studies involving 20,227 thyroid cancer cases also revealed that higher serum TSH levels were significantly associated with the size of the thyroid cancer, malignancy, and LN metastasis (32). An important finding in our study was that TSH level >1.5 mIU/L was an independent risk factor for multilevel metastasis. Although this cut-off value was not very reliable for the detection of multilevel metastasis, the sensitivity was 75.0% and specificity was 42.6%. In accordance with our findings, in the study of Jin et al., the optimal TSH concentration for LLNM prediction in patients with papillary thyroid carcinoma was 2.495 mIU/L with an AUC of 0.624, sensitivity of 56.0%, and specificity of 67.7% (33).
The present study has several limitations. First, it was a single-center retrospective study, and the sample size was small. Second, the advantages and disadvantages of the surgical techniques in this study need to be verified by long-term follow-up, including patients' quality of life and tumor recurrence rate. Therefore, multicenter cooperation and long-term follow-up are needed to obtain more reliable results.
Conclusions
In N1b PTMC patients, neck levels II–IV may be comprehensively dissected. MRND may be reserved for patients with simultaneous 3-level metastasis or clinically evident metastasis in level V. The meticulous evaluation of the lateral neck is important for N1b PTMC patients with tumors located in the upper pole or for patients with ETE or ENE. Furthermore, we should not only pay attention to the number of metastatic central LNs, but also carefully count each resected central LN, because patients with high CLNR have a higher proportion of multilevel LLNM and may need closer postoperative follow-up. Our results also suggest that high TSH levels may promote the occurrence of multilevel LLNM. USG has excellent diagnostic accuracy in evaluating multilevel LLNM in PTMC, which can help surgeons determine the appropriate extent of surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-284/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-284/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-284/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Medical Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital (No. bc2022100). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Lee CI, Kutlu O, Khan ZF, et al. Margin Positivity and Survival in Papillary Thyroid Microcarcinoma: A National Cancer Database Analysis. J Am Coll Surg 2021;233:537-44. [Crossref] [PubMed]
- Siddiqui S, White MG, Antic T, et al. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid 2016;26:807-15. [Crossref] [PubMed]
- Gao L, Li X, Xia Y, et al. Large-Volume Lateral Lymph Node Metastasis Predicts Worse Prognosis in Papillary Thyroid Carcinoma Patients With N1b. Front Endocrinol (Lausanne) 2021;12:815207. [Crossref] [PubMed]
- Huang K, Gao N, Bian D, et al. Associations of BRAF V600E, clinical pathology and imaging factors with the recurrence rate of papillary thyroid microcarcinoma. Exp Ther Med 2020;20:243. [Crossref] [PubMed]
- Kim SK, Park I, Hur N, et al. Patterns, predictive factors and prognostic impact of multilevel metastasis in N1b papillary thyroid carcinoma. Br J Surg 2017;104:857-67. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 2002;128:751-8. [Crossref] [PubMed]
- Wang W, Zhang Z, Zhao Y, et al. Management of Lateral Multiple-Level Metastasis in N1b Papillary Thyroid Microcarcinoma. Front Oncol 2020;10:1586. [Crossref] [PubMed]
- Strajina V, Dy BM, McKenzie TJ, et al. Comprehensive Lateral Neck Dissection in Papillary Thyroid Carcinoma may Reduce Lateral Neck Recurrence Rates. Ann Surg Oncol 2019;26:86-92. [Crossref] [PubMed]
- Kim SK, Park I, Hur N, et al. Should Level V Be Routinely Dissected in N1b Papillary Thyroid Carcinoma? Thyroid 2017;27:253-60. [Crossref] [PubMed]
- Xue S, Wang P, Zhang Q, et al. Routine Lateral Level V Dissection May Not Be Necessary for Papillary Thyroid Microcarcinoma With Lateral Lymph Node Metastasis: A Retrospective Study of 252 Cases. Front Endocrinol (Lausanne) 2019;10:558. [Crossref] [PubMed]
- Colakoglu B, Alis D, Seymen H. Diagnostic Accuracy of Ultrasound for the Evaluation of Lateral Compartment Lymph Nodes in Papillary Thyroid Carcinoma. Curr Med Imaging 2020;16:459-65. [Crossref] [PubMed]
- Eun NL, Kim JA, Gweon HM, et al. Preoperative Nodal US Features for Predicting Recurrence in N1b Papillary Thyroid Carcinoma. Cancers (Basel) 2021;14:174. [Crossref] [PubMed]
- American Thyroid Association Surgery Working Group. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 2009;19:1153-8. [Crossref] [PubMed]
- Yu XM, Wan Y, Sippel RS, et al. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg 2011;254:653-60. [Crossref] [PubMed]
- Likhterov I, Reis LL, Urken ML. Central compartment management in patients with papillary thyroid cancer presenting with metastatic disease to the lateral neck: Anatomic pathways of lymphatic spread. Head Neck 2017;39:853-9. [Crossref] [PubMed]
- Bhattacharyya N. Surgical treatment of cervical nodal metastases in patients with papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2003;129:1101-4. [Crossref] [PubMed]
- Yang J, Gong Y, Yan S, et al. Risk factors for level V lymph node metastases in solitary papillary thyroid carcinoma with clinically lateral lymph node metastases. Cancer Med 2016;5:2161-8. [Crossref] [PubMed]
- Kang BC, Roh JL, Lee JH, et al. Candidates for limited lateral neck dissection among patients with metastatic papillary thyroid carcinoma. World J Surg 2014;38:863-71. [Crossref] [PubMed]
- Song K, Jin Y, Kim M, et al. Patterns of Occult Metastasis to Level Va and Vb in Clinically Lateral Node-Positive Papillary Thyroid Carcinoma. Ann Surg Oncol 2022;29:2550-6. [Crossref] [PubMed]
- Tong Y, Li J, Huang Y, et al. Ultrasound-Based Radiomic Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Acad Radiol 2021;28:1675-84. [Crossref] [PubMed]
- Li C, Meng ZZ, Qin JW, et al. Analysis of Risk Factors of Level V Lymphatic Metastasis for Papillary Thyroid Carcinoma with pN1b. J Oncol 2021;2021:5562065. [Crossref] [PubMed]
- Feng JW, Qin AC, Ye J, et al. Predictive Factors for Lateral Lymph Node Metastasis and Skip Metastasis in Papillary Thyroid Carcinoma. Endocr Pathol 2020;31:67-76. [Crossref] [PubMed]
- Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer 2019;19:622. [Crossref] [PubMed]
- Heng Y, Feng S, Yang Z, et al. Features of Lymph Node Metastasis and Structural Recurrence in Papillary Thyroid Carcinoma Located in the Upper Portion of the Thyroid: A Retrospective Cohort Study. Front Endocrinol (Lausanne) 2021;12:793997. [Crossref] [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg 2004;28:1115-21. [Crossref] [PubMed]
- Carvalho AY, Kohler HF, Gomes CC, et al. Predictive Factors of Recurrence of Papillary Thyroid Microcarcinomas: Analysis of 2,538 Patients. Int Arch Otorhinolaryngol 2021;25:e585-93. [Crossref] [PubMed]
- Kou Y, Shen G, Cheng Z, et al. Predictive Value of Gross Extranodal Extension for Differentiated Thyroid Carcinoma Persistence/Recurrence. Otolaryngol Head Neck Surg 2022;166:643-51. [Crossref] [PubMed]
- Mao A, An N, Wang J, et al. Association between preoperative serum TSH and tumor status in patients with papillary thyroid microcarcinoma. Endocrine 2021;73:617-24. [Crossref] [PubMed]
- Tam AA, Ozdemir D, Aydın C, et al. Association between preoperative thyrotrophin and clinicopathological and aggressive features of papillary thyroid cancer. Endocrine 2018;59:565-72. [Crossref] [PubMed]
- Zheng J, Li C, Lu W, et al. Quantitative assessment of preoperative serum thyrotropin level and thyroid cancer. Oncotarget 2016;7:34918-29. [Crossref] [PubMed]
- Jin S, Bao W, Yang YT, et al. Establishing a prediction model for lateral neck lymph node metastasis in patients with papillary thyroid carcinoma. Sci Rep 2018;8:17355. [Crossref] [PubMed]
(English Language Editor: K. Brown)


