
Escaping sestamibi detection: a case report of aggressive and recurrent metastatic parathyroid carcinoma
Introduction
Parathyroid carcinoma is exceedingly rare, representing only 0.005% of all cancers. This cancer does not metastasize in a predictable manner, as it has been noted to spread both hematogenously and lymphatically (1,2). Although metastasis to ipsilateral levels III, IV, and V cervical lymph nodes are commonly seen, there are currently no cases in the literature that report metastasis to a contralateral level II lymph node. Furthermore, this locus of metastasis was undetectable by nuclear medicine (NM) sestamibi scan. We present the following case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-9/rc).
Case presentation
Our patient is a 32-year-old man with an 8-year history of renal stones and lithotripsy who presented to the emergency department for painless hematuria. On exam, he was found to be in hypertensive urgency (204/119 mmHg), and his urine was blood tinged. Otherwise, physical exam and review of systems were negative. He denied abdominal pain, constipation, fatigue, or changes in weight or appetite. Patient reported occasional alcohol use but no smoking or illicit substance use. Family history was negative for heart disease, strokes, diabetes, or endocrine/thyroid pathology.
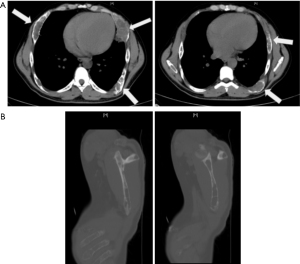
He was found to have an acute on chronic renal insufficiency with creatinine of 2.4 mg/dL (2.1 mg/dL 5 years prior). Furthermore, he was found to have parathyroid hormone (PTH) level of 2,165 pg/mL, severely decreased vitamin D (<4 ng/mL), and elevated calcium (11.9, down to 10.4 mg/dL after hydration). Thyroid function tests were notable for low free T4 (0.55 ng/dL) but were otherwise unremarkable. Computed tomography (CT) scans revealed lytic bone lesions of the clavicle, scapula, and humorous (Figure 1). Ultimately, he was admitted for hypertensive emergency and workup of lytic bone lesions. Iliac bone biopsy revealed giant cell tumors consistent with primary hyperparathyroidism, and ultrasound (US) revealed a vascular, right-sided neck mass 2.1 cm × 1.9 cm × 2.8 cm (Figure 2). Sestamibi scan demonstrated hyperactivity on the right side of the neck. Differential diagnosis for primary hyperparathyroidism included parathyroid adenoma; however, based on the severity of hyperparathyroidism (PTH above 2,000 pg/mL with extensive lytic lesions) and a low T4 level, parathyroid carcinoma was also suspected.
Outpatient US revealed lymphadenopathy suspicious for malignancy, and fine needle aspiration (FNA) of the right sided neck mass returned as a cystic lesion with scattered macrophages and cholesterol crystals; PTH washout was 30,000 pg/mL. Patient underwent subsequent en bloc resection of parathyroid carcinoma, right-sided lobectomy, and central lymph node dissection due to increased suspicion for parathyroid carcinoma.
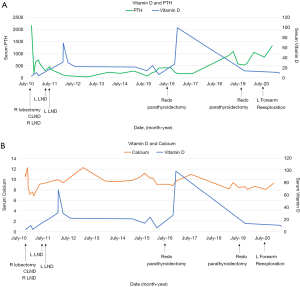
After initially feeling more energetic after surgery, he began to experience fatigue. Over the next 3 months, serum PTH levels continued to be elevated (495–735 pg/mL). Neck US revealed a suspicious 1.6 cm level II lymph node, but this time on the left side. FNA revealed elevated PTH washout, and the patient underwent two left lateral neck dissections one month apart for a radiologically suspicious left level II lymph node. Neither dissection revealed metastatic carcinoma in 26 lymph nodes. Over the next 4 years subsequent labs revealed improved PTH and calcium, but levels never normalized (Figure 3). Patient underwent a repeat sestamibi scan, and no evidence of abnormal parathyroid radiotracer update was detected. Throughout this period, the patient was prescribed sevelamer (2,400 mg TID), cinacalcet (60 mg/day), and vitamin D (0.25 mcg/day) for symptoms of hyperparathyroidism. The patient was consistently adherent to medications and tolerated them well.
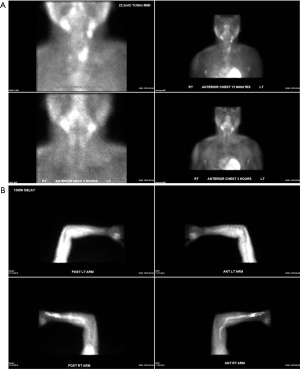
Despite pharmacological intervention, the patient experienced worsening fatigue and elevated PTH. In the following 3 years he underwent two redo parathyroidectomies as well as autotransplantation of parathyroid tissue to the left brachioradialis. Two sestamibi scans were performed, including of the left forearm autotransplantation site, both of which noted no abnormal uptake (Figure 4). Fluorodeoxyglucose-positron emission tomography (FDG-PET) scan was not available at this hospital. Re-exploration of the left forearm parathyroid tissue revealed benign parathyroid tissue.
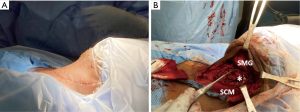
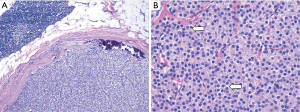
Neck US one decade after initial diagnosis demonstrated a suspicious, vascular level II lymph node measuring 2.2 cm × 1.61 cm × 1.22 cm on the contralateral (left) side (Figure 5). FNA noted parathyroid tissue, and PTH aspirate was 7,180 pg/mL. This patient underwent left modified radical neck dissection (MRND) (Figure 6). During surgery, intraoperative PTH dropped from 316.4 to 132.9 pg/mL. Surgical pathology confirmed recurrent metastatic parathyroid carcinoma involving thymic tissue (Figures 7,8A,8B). Two weeks post-operatively, his PTH had decreased to 585 pg/mL. He continues to take sevelamer (800 mg TID), calcitriol (0.25 mg/day), and levothyroxine (75 mcg/day), but is no longer taking sensipar. Fortunately, the only postoperative complication through one decade of treatment was hypothyroidism; vocal cords appeared normal. Update: at 6-month follow-up, the patient did not demonstrate biochemical recurrence.
This case is further complicated by chronic kidney disease that eventually progressed to end stage kidney disease. Therefore, calcium levels are often inappropriately within an acceptable range with medication. It is also worth noting that this patient underwent hemicolectomy for pT1N0M0 colon cancer causing cecal obstruction after initial diagnosis of parathyroid carcinoma. Given the development two cancers at a young age, the absence of family history of cancer, the absence of known carcinogenic exposures, and the presence of a rare cancer, there is concern for a genetic cancer syndrome. The combination of colon cancer and parathyroid carcinoma is not seen in any known cancer syndrome, but the patient was nevertheless offered a genetic panel for a variety of hereditary cancer syndromes, which are pending.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Parathyroid carcinoma is exceedingly rare, representing only 0.005% of all cancers (1). Due to the difficulty of clinically distinguishing between parathyroid carcinoma and adenoma, diagnosis is most often reached via surgical pathology (3).
Parathyroid carcinoma recurrence is common and presents as increasing PTH and serum calcium. Although the estimation varies, the recurrence rate is most often cited as about 50% (1,3). Monitoring for occurrence is done via serum PTH and calcium, or via sestamibi PET-CT scans when progressive disease is suspected. Although not generally recommended due to risk of rupture or metastasis, FNA of suspicious neck lesions can be performed for cytologic diagnosis and PTH washout (4).
Morbidity and mortality of parathyroid carcinoma result from hypercalcemia (e.g., bone pain, psychiatric disturbances, renal calculi) and subsequent end organ failure (4). Treatment most often is metastasectomy, with denosumab or sensipar to control the effects of hypercalcemia. Radiofrequency ablation (RFA) and immunotherapy have also been used in treatment (1).
The mainstay of treatment for metastatic parathyroid carcinoma is metastasectomy (1), but this is only possible if the metastasis can be localized. This case exemplifies how unpredictable metastases in this disease can be, and how several techniques may be necessary to identify the source of recurrent disease. Over time the reported sensitivity of sestamibi in detecting parathyroid neoplasms has decreased from 80–100% (5) to 58–78% (6). A 2017 study of 20 patients found that the sensitivity and accuracy of sestamibi was 81% and 78%, respectively, when used specifically for parathyroid carcinoma (7). It would make sense that parathyroid carcinoma demonstrates a higher specificity than adenoma, as some research suggests that parathyroid carcinoma demonstrates a higher retention level of 99mTc-labeled sestamibi (8); however, this was not the case for this patient.
In cases where a sestamibi scan is not able to localize a metastatic focus in a patient with known parathyroid carcinoma, FNA and PTH washout are important secondary studies. Although FNA is unlikely to become the gold standard of diagnosis because of its associated risks (i.e., parathyromatosis, seeding, or rupture of the tumor), it was an invaluable diagnostic adjunct for localization of metastasis in this patient (1). Additionally, the clinician must consider the role of anatomy; a case report of metastatic parathyroid carcinoma from 2003 described unusual lymphatic spread to the retropharyngeal lymph nodes. In this case, US was not able to detect the metastasis due to the deep location of the nodes (9). However, in our case, FNA with PTH washout was the only diagnostic armamentarium useful to establish diagnosis of recurrent metastatic disease.
Several aspects of this case make an already rare diagnosis more exceptional. While most parathyroid carcinomas metastasize to ipsilateral lymph nodes at levels III, IV, and V, this case metastasized to a contralateral level II node. This cancer was aggressive and recurrent, able to metastasize from the right to the left neck despite an extensive surgical history. Of particular mystery is the suspicious left lateral level II lymph node that triggered the initial lateral neck dissections (2nd paragraph of Case presentation). This lymph node was possibly a false positive, or it was only one of several metastatic focuses and others were either not detected and/or not entirely resected. In the latter case, this would further demonstrate the resilience and aggression of this tumor. Regardless, this aggressive and unexpected tumor exemplifies the unpredictability of parathyroid carcinoma metastases; close monitoring and a multifaceted approach may be necessary in a patient with recurrent disease.
Furthermore, the young age of this patient and the histology of the tumor (diffuse and sheet-like with paranuclear clearing, Figure 8B) raises suspicion for a parafibromin-deficient neoplasm associated with a CDC73 mutation. Erickson et al. (10) in 2022 have recommended regular parafibromin immunohistochemistry for atypical parathyroid tumors and parathyroid carcinomas to assess recurrence risk, and if the tumor is parafibromin negative, CDC73 gene sequencing to assess for an underlying germline mutations. Unfortunately, parafibromin immunohistochemistry is not yet readily available at our institution. Regardless of parafibromin status, this tumor had already demonstrated itself at the time to be aggressive, be recurrent, and require extensive follow-up. However, the patient is scheduled to be treated for a variety of inborn genetic cancer syndromes, including but not limited to familial adenomatous polyposis and those resulting from a CDC73 mutation. It is at this time unclear whether this patient has an underlying genetic disorder uniting the two instances of cancer at such a young age (e.g., familial adenomatous polyposis or Lynch syndrome presenting as parathyroid neoplasm) (11). In a Swedish study with over 90,000 person-years of observation, simply the presence of primary hyperparathyroidism was associated with an increased risk of colon cancer (12).
This patient was further complicated by end stage renal disease and secondary hyperparathyroidism that developed during treatment for parathyroid carcinoma; therefore, calcium levels were deceptively low, and PTH is unlikely to completely normalize. The patient’s inability to produce excessive calcium may also explain why, despite extreme elevations in PTH, the patient’s symptoms were mild and limited to fatigue.
Acknowledgments
The authors would like to acknowledge the Tulane University Department of Pathology and Laboratory Medicine for their assistance in obtaining histologic and gross pathology.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-9/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-9/coif). EK serves as the editor-in chief of Gland Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goswamy J, Lei M, Simo R. Parathyroid carcinoma. Curr Opin Otolaryngol Head Neck Surg 2016;24:155-62. [Crossref] [PubMed]
- Marini F, Giusti F, Palmini G, et al. Genetics and Epigenetics of Parathyroid Carcinoma. Front Endocrinol (Lausanne) 2022;13:834362. [Crossref] [PubMed]
- Mohebati A, Shaha A, Shah J. Parathyroid carcinoma: challenges in diagnosis and treatment. Hematol Oncol Clin North Am 2012;26:1221-38. [Crossref] [PubMed]
- Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol 2012;13:11-23. [Crossref] [PubMed]
- Shaha AR, Sarkar S, Strashun A, et al. Sestamibi scan for preoperative localization in primary hyperparathyroidism. Head Neck 1997;19:87-91. [Crossref] [PubMed]
- Tay D, Das JP, Yeh R. Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review. Biomedicines 2021;9:390. [Crossref] [PubMed]
- Christakis I, Vu T, Chuang HH, et al. The diagnostic accuracy of neck ultrasound, 4D-Computed tomography and sestamibi imaging in parathyroid carcinoma. Eur J Radiol 2017;95:82-8. [Crossref] [PubMed]
- Zhang M, Sun L, Rui W, et al. Semi-quantitative analysis of 99mTc-sestamibi retention level for preoperative differential diagnosis of parathyroid carcinoma. Quant Imaging Med Surg 2019;9:1394-401. [Crossref] [PubMed]
- Rufener JB, Cohen JI. Metachronous spread of parathyroid carcinoma to a retropharyngeal lymph node. Head Neck 2003;25:968-71. [Crossref] [PubMed]
- Erickson LA, Mete O, Juhlin CC, et al. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr Pathol 2022;33:64-89. [Crossref] [PubMed]
- Andreasson A, Sulaiman L, do Vale S, et al. Molecular characterization of parathyroid tumors from two patients with hereditary colorectal cancer syndromes. Fam Cancer 2012;11:355-62. [Crossref] [PubMed]
- Nilsson IL, Zedenius J, Yin L, et al. The association between primary hyperparathyroidism and malignancy: nationwide cohort analysis on cancer incidence after parathyroidectomy. Endocr Relat Cancer 2007;14:135-40. [Crossref] [PubMed]





