
Frozen section analysis of central lymph nodes in papillary thyroid cancer: the significance in determining the extent of surgery
Introduction
Surgical treatment strategies for differentiated thyroid cancer have consistently changed. Over the last decade, a worldwide increase in thyroid cancer incidence has been observed, especially in papillary thyroid cancer (PTC), with indolent features and a better prognosis compared with other cancers. This has led to a gradual increase in less aggressive treatment options in preference to total thyroidectomies (TTs) (1,2).
The appropriate extent of thyroid surgery in patients with low-risk PTC remains controversial. Cervical lymph node (LN) metastasis is a significant consideration for determining the extent of surgery. LN metastasis occurs in 40–90% of PTC cases and is an important prognostic factor of local recurrence; proper diagnosis and removal of metastatic LNs are critical in treating PTC (3,4). However, not all LN metastases are identified preoperatively because of the limited diagnostic performance of ultrasonography (US) and computed tomography (CT) (5), especially if they are small-volume metastases or are placed in an unreachable location of the neck.
LN metastasis of PTC primarily occurs in the central compartment of the neck, which is referred to as neck level VI. Several guidelines suggest various nodal criteria for determining the surgical extent of PTC, but they all represent the current trends in less-aggressive surgery. The National Comprehensive Cancer Network (NCCN) guidelines version 3.2021 recommend withholding the completion thyroidectomy for incidental small-volume pathologic N1a metastasis: <5 involved LNs with no metastasis >2 mm in the largest dimension (6).
In our institution, we have performed elective ipsilateral central compartment neck dissection (CCND) in patients with clinically node-negative (cN0) PTC, as well as therapeutic CCND in clinically node-positive (cN1a) disease. Despite the controversies regarding prophylactic CCND for PTC and the risk of surgical morbidities (hypocalcemia, recurrent laryngeal nerve palsy, etc.), we prefer elective ipsilateral CCND because of the benefit of therapeutic removal of occult metastatic LNs and accurate LN staging (7). Moreover, we conducted frozen analysis on the resected central LNs to obtain nodal information and confirm the proper surgical extent intraoperatively, thereby avoiding secondary surgery for completion thyroidectomy in cases with unexpectedly extensive LN metastases (8).
In this study, we retrospectively reviewed and reported our experience with elective CCND with frozen analysis of unilateral cN0 PTC. First, we presented our nodal criteria for determining the surgical extent and compared the clinicopathologic features of patients who underwent TT with those who had less than TT (LTT). Second, we categorized the patients based on the nodal criteria of the NCCN guidelines and assessed the clinicopathologic differences. Third, we investigated patients who showed different frozen and permanent results, and the diagnostic accuracy of the frozen analysis was assessed. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-15/rc).
Methods
Patients
A total of 750 consecutive patients with PTC underwent thyroid surgery at the Department of Surgery, Thyroid Cancer Center of CHA Ilsan Medical Center, CHA University College of Medicine, from March 2020 to May 2021. Of these, 290 (38.7%) had cN0 unilateral PTC and underwent elective ipsilateral CCND. Intraoperative frozen analyses were performed for central LNs. Patients with bilateral cancers or contralateral suspicious nodules, clinically node-positive disease, cancer with gross strap muscle invasion, non-malignant lesions, and other malignancies were excluded from the study. The medical records of the included patients were retrospectively reviewed.
Preoperative work-up
The preoperative patient work-up included physical examination, high-resolution US, and thorcocervical CT. All patients were preoperatively diagnosed with PTC (Bethesda category VI) or suspicious PTC (Bethesda category V) based on US-guided fine-needle aspiration biopsy. The size and location of the tumor, degree of extrathyroidal extension, nodal involvement, and other abnormal findings in the neck and chest were complementarily evaluated using the preoperative US and thoracocervical CT. Clinical LN metastasis (cN1a) was defined as a pathologic change within the LN noted in both evaluations.
Surgery
Hemithyroidectomy with elective ipsilateral CCND was primarily performed in all patients. If contralateral benign-looking nodules were present in the accessible region, contralateral partial thyroidectomy was also considered. The patients who received hemithyroidectomy or hemithyroidectomy with contralateral partial thyroidectomy were categorized into those who had “LTT”. The central compartment LNs consist of the neck level 6 LNs (pretracheal, prelaryngeal, and paraesophageal LNs), which include the LNs from the hyoid bone superior to the innominate (brachiocephalic) artery inferiorly. On each side, the lateral boundary was limited by the medial border of the carotid sheath. Harvested central LNs were sent for frozen analysis.
The extent of surgery was primarily determined by the maximal size of the metastatic LNs. To determine the surgical extent, we set our own cut-off of nodal size as “5 mm” in the longest diameter. Completion thyroidectomy was performed in cases with LN metastasis of ≥5 mm. There was no specific criterion for the number of metastatic LNs in determining completion thyroidectomy.
Pathology
Histopathological examination of the resected central LNs was performed using frozen and permanent analysis. Frozen section analyses were performed for the central LNs in all patients. For frozen analysis, two 3–4-µm-thick cryosection samples were cut in the microtome and inspected within 30 min. The results were instantly reported in the number of metastatic LNs excised to the total number of removed LNs, with maximal diameter of the metastatic LN. Permanent pathology was confirmed within 2 weeks after a thorough examination. Three pathologists with expertise in thyroid diseases were involved in the frozen and permanent analyses, as scheduled.
Micrometastasis was defined as maximum metastatic focus size <0.2 cm, while ≥0.2 cm was defined as macrometastasis. The LN ratio (LNR) was defined as the ratio of the number of positive LNs excised to the total number removed. Papillary microcarcinoma (PMC) was diagnosed as PTC with a tumor diameter of <1 cm. Hashimoto thyroiditis was diagnosed with the typical histologic findings of extensive lymphocytic infiltrate with the germinal center formation with occasional tissue fibrosis and appearance of abundant Hürthle cells or oncocytes. TNM classification system of the American Joint Committee on Cancer (AJCC) eighth edition was used for the staging.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of CHA Ilsan Medical Center (2021-11-005), and individual consent for this retrospective analysis was waived.
Statistical analysis
Categorical data are reported as rates and proportions, while the median and range were calculated for continuous data. Differences in continuous variables were compared using the Mann-Whitney test or Student’s t-test, and differences in categorical variables were compared using the Chi-square (χ2) test or Fisher’s exact test, as appropriate. Statistical significance was set at P<0.05. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of intraoperative frozen analyses were examined based on the permanent pathology results. We further measured the differences between the frozen and permanent results using the χ2 test. All data were processed and statistically analyzed using IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY, USA).
Results
Analyses of patients according to the surgical extent
The baseline clinicopathological characteristics of the 290 patients are shown in Table 1. After hemithyroidectomy with elective CCND, frozen analyses of the resected central LNs were performed. Among them, completion TT was indicated in 47 (16.2%) patients.
Table 1
| Characteristics | Value |
|---|---|
| Number of patients | 290 |
| Age (years), mean ± SD [range] | 42.0±10.69 [14–77] |
| Sex, M:F (F%) | 60:230 (79.3) |
| Type of surgery, n (%) | |
| Hemi + CCND | 199 (68.6) |
| Hemi + contralateral partial + CCND | 44 (15.2) |
| TT + CCND | 47 (16.2) |
| Operation time (minutes), mean ± SD [range] | 87.4±25.5 [40–180] |
| Tumor size (cm), mean ± SD [range] | 0.9±0.7 [0.9–4.7] |
| Extrathyroidal extension, n (%) | 67 (23.1) |
| Multiplicity, n (%) | 57 (19.7) |
| Hashimoto thyroiditis, n (%) | 151 (52.1) |
| Pathology, n (%) | |
| Conventional | 267 (92.2) |
| Follicular variant | 9 (3.1) |
| Diffuse sclerosing variant | 6 (0.5) |
| Tall cell variant | 12 (4.1) |
| Oncocytic variant | 1 (0.3) |
| Solid and trabecular variant | 1 (0.3) |
| Frozen analyses, mean ± SD [range] | |
| Number of retrieved LNs | 4.2±3.0 [0–19] |
| Number of metastatic LNs | 1.0±1.7 [0–9] |
| Metastatic LN size (max, cm) | 0.3±0.3 [0.005–1.4] |
| Permanent analyses, mean ± SD [range] | |
| Number of retrieved LNs | 4.8±3.6 [0–22] |
| Number of metastatic LNs | 1.2±1.8 [0–10] |
| Metastatic LN size (max, cm) | 0.3±0.3 [0–1.4] |
| Extranodal extension, n (%) | 17 (5.9) |
| TNM classification, n (%) | |
| T stage, T1/2/3/4 | 217 (74.9)/58 (20.0)/10 (3.4)/5 (1.7) |
| N stage, N0/1a | 152 (52.4)/138 (47.6) |
| M stage, M0/1 | 290 (100.0)/0 (0.0) |
| Stage I/II/III/IV | 258 (89.0)/30 (10.3)/2 (0.7)/0 (0.0) |
cN0, clinically nodal negative; PTC, papillary thyroid carcinoma; M, male; F, female; hemi, hemithyroidectomy; CCND, central compartment neck dissection; contralateral partial, contralateral partial thyroidectomy; TT, total thyroidectomy; LN, lymph node; max, maximum.
We evaluated the patients according to the surgical extent as the LTT and TT groups (Table 2). Comparative analyses revealed that the mean tumor size was larger (0.8±0.5 vs. 1.4±0.9 cm, P<0.001), and fewer females (81.9% vs. 66.0%, P<0.001) were involved in the TT group. The mean total operation time was longer in the TT group (82.4±22.0 vs. 113.3±26.5 minutes, P<0.001).
Table 2
| Characteristics | LTT | TT | P value |
|---|---|---|---|
| Number of patients | 243 | 47 | |
| Age (years), mean ± SD [range] | 42.2±10.3 [25–77] | 40.6±12.5 [14–75] | 0.339 |
| Sex, M:F (F%) | 44:199 (81.9) | 16:31 (66.0) | 0.014 |
| Operation time (minutes), mean ± SD [range] | 82.4±22.0 [50–155] | 113.3±26.5 [55–180] | <0.001 |
| Tumor size (cm), mean ± SD [range] | 0.8±0.5 [0.1–3.4] | 1.4±0.9 [0.3–4.7] | <0.001 |
| Extrathyroidal extension, n (%) | 51 (21.0) | 16 (34.0) | 0.141 |
| Multiplicity, n (%) | 42 (17.3) | 15 (31.9) | 0.065 |
| Hashimoto thyroiditis, n (%) | 126 (51.9) | 25 (53.2) | 0.866 |
| Pathology, n (%) | 0.879 | ||
| Conventional | 222 (91.8) | 44 (93.6) | |
| Follicular variant | 7 (2.9) | 2 (4.3) | |
| Diffuse sclerosing variant | 0 (0.0) | 0 (0.0) | |
| Tall cell variant | 11 (4.5) | 1 (2.1) | |
| Oncocytic variant | 1 (0.4) | 0 (0.0) | |
| Solid and trabecular variant | 1 (0.4) | 0 (0.0) | |
| Frozen analyses, mean ± SD [range] | |||
| Number of retrieved LNs (A) | 3.9±2.9 [0–19] | 5.4±3.3 [1–15] | 0.002 |
| Number of metastatic LNs (B) | 0.7±1.2 [0–6] | 2.7±2.7 [0–9] | <0.001 |
| Metastatic LN size (max, cm) | 0.1±0.2 [0.005–0.4] | 0.7±0.3 [0.2–1.4] | <0.001 |
| LNR (A/B) | 0.2±0.3 [0–1.0] | 0.5±0.4 [0.005–1.0] | <0.001 |
| Permanent analyses, mean ± SD [range] | |||
| Number of retrieved LNs | 4.5±3.5 [0–22] | 6.3±3.8 [1–18] | 0.002 |
| Number of metastatic LNs | 0.8±1.3 [0–7] | 3.0±2.8 [0–10] | <0.001 |
| Metastatic LN size (max, cm) | 0.3±0.2 [0.005–0.8] | 0.6±0.3 [0.1–1.4] | <0.001 |
| Extranodal extension, n (%) | 8 (7.8) | 9 (25.0) | 0.007 |
| LNR (A/B) | 0.2±0.3 [0–1.0] | 0.5±0.4 [0–1.0] | <0.001 |
| TNM classification, n (%) | |||
| T stage, T1/2/3/4 | 198 (81.5)/39 (16.0)/2 (0.8)/4 (1.6) | 19 (40.4)/19 (40.4)/8 (17.0)/1 (2.1) | <0.001 |
| N stage, N0/1a | 138 (57.7)/101 (42.3) | 0 (0.0)/47 (100.0) | <0.001 |
| Stage I/II/III | 225 (92.6)/18 (7.4)/0 (0.0) | 3 (70.2)/12 (25.5)/2 (4.3) | <0.001 |
LTT, less than total thyroidectomy; TT, total thyroidectomy; M, male; F, female; LN, lymph node; max, maximum; LNR, LN ratio; SD, standard deviation.
A significant difference was noted for all nodal characteristics. According to frozen analyses, the mean number of retrieved (3.9±2.9 vs. 5.4±3.3, P=0.002) and metastatic LNs (0.7±1.2 vs. 2.7±2.7, P<0.001) was larger in the TT group. Moreover, the LNR was higher (0.2±0.3 vs. 0.5±0.4, P<0.001) and the size of maximal metastatic LN was larger (0.2±0.3 vs. 0.7±0.3, P<0.001) in the TT group. Similar findings with more extranodal extension (ENE) (7.8% vs. 25.0%, P=0.007) were observed in the permanent analyses. This led to more N1a stages in the TT group (42.3% vs. 100%, P<0.001) (Table 2).
There were no significant differences in other tumor factors, such as extrathyroidal extension, tumor multiplicity, the presence of Hashimoto thyroiditis, and variants of PTC.
Analyses of patients according to the nodal criteria of NCCN guidelines
We classified the patients into two groups based on the nodal criteria. The first group consisted of patients who did not require completion TT: those with <5 involved central LNs with metastasis ≤2 mm in the largest dimension. The others who did not meet the criteria were categorized as the second group. According to the NCCN guidelines, completion TT was indicated in 72 (24.8%) patients and included in the second group (Table 3).
Table 3
| Characteristics | <5 (+) LNs, and ≤2 mm | ≥5 (+) LNs, or >2 mm | P value |
|---|---|---|---|
| Number of patients | 218 | 72 | |
| Age (years), mean ± SD [range] | 43.0±10.7 [14–77] | 38.9±10.2 [17–75] | 0.005 |
| Sex, M:F (F%) | 32:186 (85.3) | 28:44 (59.5) | <0.001 |
| Tumor size (cm), mean ± SD [range] | 0.8±0.6 [0.1–4.7] | 1.1±0.5 [0.4–2.6] | 0.004 |
| Extrathyroidal extension, n (%) | 46 (21.1) | 21 (29.2) | 0.322 |
| Multiplicity, n (%) | 40 (18.3) | 17 (23.6) | 0.536 |
| Hashimoto thyroiditis, n (%) | 117 (53.7) | 34 (47.2) | 0.342 |
| Pathology, n (%) | 0.494 | ||
| Conventional | 201 (92.2) | 66 (91.7) | |
| Follicular variant | 8 (3.7) | 1 (1.4) | |
| Diffuse sclerosing variant | 0 (0.0) | 0 (0.0) | |
| Tall cell variant | 7 (3.2) | 5 (6.9) | |
| Oncocytic variant | 1 (0.5) | 0 (0.0) | |
| Solid and trabecular variant | 1 (0.5) | 0 (0.0) | |
| Frozen analyses, mean ± SD [range] | |||
| Number of retrieved LNs (A) | 3.9±2.9 [0–19] | 5.0±3.3 [1–15] | 0.006 |
| Number of metastatic LNs (B) | 0.4±0.7 [0–4] | 3.0±2.1 [0–9] | <0.001 |
| Metastatic LN size (max, cm) | 0.04±0.08 [0.005–0.2] | 0.5±0.3 [0.2–1.4] | <0.001 |
| LNR (A/B) | 0.1±0.2 [0–1.0] | 0.7±0.3 [0.005–1.0] | <0.001 |
| Permanent analyses, mean ± SD [range] | |||
| Number of retrieved LNs (A) | 4.5±3.5 [0–22] | 5.8±3.9 [1–18] | 0.007 |
| Number of metastatic LNs (B) | 0.4±0.7 [0–4] | 3.4±2.3 [1–10] | <0.001 |
| Metastatic LN size (max, cm) | 0.05±0.08 [0.005–0.3] | 0.5±0.3 [0.2–1.4] | <0.001 |
| Extranodal extension, n (%) | 4 (6.1) | 13 (18.1) | 0.032 |
| LNR (A/B) | 0.1±0.2 [0–1.0] | 0.7±0.3 [0–1.0] | <0.001 |
| TNM classification, n (%) | |||
| T stage, T1/2/3/4 | 176 (80.7)/33 (15.1)/5 (2.3)/4 (1.8) | 41 (56.9)/25 (34.7)/5 (6.9)/1 (1.4) | <0.001 |
| N stage, N0/1a | 147 (68.7)/67 (31.3) | 0 (0.0)/72 (100) | <0.001 |
| Stage I/II/III | 200 (91.7)/16 (7.3)/2 (1.0) | 58 (80.6)/14 (19.4)/0 (0.0) | 0.011 |
NCCN, National Comprehensive Cancer Network; (+), positive; M, male; F, female; LN, lymph node; max, maximum; LNR, LN ratio; SD, standard deviation.
Comparative analyses revealed that the patients in the second group were younger (43.0±10.7 vs. 38.9±10.2, P=0.005), consisted of more men (85.3% vs. 59.5%, P<0.001), and had bigger tumors (0.8±0.6 vs. 1.1±0.5, P=0.004).
Nodal characteristics were significantly different between the two groups. Frozen analyses showed more retrieved LNs (3.9±2.9 vs. 5.0±3.3, P=0.006) and metastatic LNs (0.4±0.7 vs. 3.0±2.1, P<0.001) in the second group. Moreover, the LNR was higher (0.1±0.2 vs. 0.7±0.3, P<0.001) and the size of maximal metastatic LN was larger (0.04±0.08 vs. 0.5±0.3, P<0.001) in the second group. Permanent analyses revealed similar findings with more ENE (6.1% vs. 18.1%, P=0.032). This led to more N1a stages in the TT group (31.3% vs. 100%, P<0.001) (Table 3).
Analyses of patients according to the frozen and permanent results of central LNs
Since the frozen and permanent results of central LNs were not identical, we assessed the patients with different frozen and permanent results and compared them with those with identical results. Different frozen and permanent results of the central LNs were observed in 79 patients, who tended to be younger (43.0±11.3 vs. 39.2±8.2, P=0.002).
In fact, more LNs were found, and additional resection was improvised after frozen analyses (4.0±3.0 vs. 6.3±3.8, P<0.001). More metastatic LNs (0.9±1.7 vs. 1.7±2.1, P=0.005) were identified in the permanent analyses in these patients. The N1a stage was also more frequent (43.7% vs. 60.3%, P=0.013) (Table S1).
Analyses of patients with discrepant frozen and permanent results of central LNs
We further investigated the cause of this discrepancy in these patients. First, additional LN retrieval was performed in 74 patients (93.7%). More negative LNs were excised in 49 (62.0%) patients, with an average of 2.2±1.6 (range, 1–6). Meanwhile, the positive LNs were additionally resected in 25 (31.7%) patients, with a mean number of 2.2±1.5 (range, 1–6). Among them, a larger metastatic LN was further identified during additional retrieval in 9 patients. Mean differences in the maximal diameter of metastatic LNs between frozen and permanent analyses were measured 0.2±0.1 cm (range, 0.1–0.5 cm).
Lastly, the converted result of LNs in the permanent analyses, from negative to positive, was observed in 5 (6.3%) patients. The mean number of LNs with discrepant results was 1.2±0.4, and the mean diameter of these LNs was 0.04±0.05 cm (range, 0–0.1 cm). None of the positive LNs in the frozen analyses were confirmed to be negative in the final report.
Among 30 (38.0%) patients with more positive LNs in the final report, 10 (12.7%) patients turned N0 into the N1a stage in permanent analyses. These patients comprised eight (10.1%) patients with additional positive LN retrieval and 2 (2.5%) patients with converted LN results. Their frozen results were negative from malignancy, but the mean number of 1.1±0.3 (range, 1–2) positive LNs were detected during the histopathologic examination. The mean size was reported 0.2±0.1 cm (range, 0.1–0.3 cm) (Figure 1).
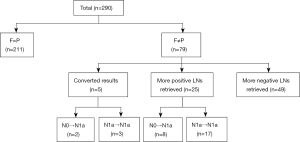
Among 243 patients who had LTT, 5 (2.1%) patients had discrepant frozen and permanent results. Additional LNs were retrieved in two patients after frozen analyses, which were found with larger metastatic LNs (maximum diameter of 0.8 and 0.7 cm). In three patients, the reported maximum diameter of metastatic LN has been changed through histopathologic examination (maximum diameter of 0.5 cm).
Diagnostic accuracy of frozen analyses of the central LN
After excluding 74 patients with additional LN retrieval, the diagnostic accuracy of frozen analyses for central LNs was evaluated in 216 patients. The sensitivity, specificity, PPV, and NPV were 94.6% (88/93), 100% (123/123), 100% (88/88), and 96.1% (123/128), respectively. Statistical significance was reached, and the diagnostic agreement rate between the frozen analyses and the permanent pathologic report was very high (κ index =0.962). The likelihood ratio was 23.57 (P<0.001).
Surgical outcomes
Among the 47 patients who had TT, 5 (10.6%) showed transient postoperative hypocalcemia, and 1 (2.1%) patient experienced transient hoarseness. None of the patients experienced permanent complications from surgery. No additional surgery of completion TT was planned in any patients who had LTT. The close observation was planned for 5 patients with discrepant frozen and permanent results. Adjuvant radioactive iodine therapy was administered to 28 patients (59.6%). The mean follow-up period was 12.4±4.0 months (range, 5–19 months). Disease recurrence was not observed in any of the patients.
Discussion
Since LN metastasis occurs in up to 90% of patients with PTC, LN metastasis has become one of the critical indicators in determining the appropriate surgical extent (3). TT with both CCNDs was preferred in patients with PTC with central LN metastasis in the past. There have been various studies about central LN metastasis on survival and recurrence, but it seems certain that proper cervical LN dissection promotes higher recurrence-free survival (4,9-12).
Several guidelines recommend an adequate therapeutic range for thyroidectomy with respect to LN metastasis (13). They are not inconsistent but are constantly changing to perform less aggressive surgery for small-volume metastasis. According to the 2015 American Thyroid Association risk stratification system guidelines, all involved LNs sized <0.2 cm and <5 metastatic LNs were classified as low-risk LN disease, whereas >5 metastatic LNs, macroscopic LN metastasis, and ≥3 cm metastatic LNs were considered as high-risk LN disease (14,15). The NCCN guidelines also recommend that TT is not indicated in <5 involved LNs with no metastasis >2 mm in the largest dimension (6).
With the development of high-resolution imaging modalities in the neck US and CT, preoperative assessment of clinical staging in PTC has become more feasible. However, there are still limitations in detecting and discriminating metastatic LNs, especially in the central LNs located in the inaccessible region or in those with micrometastases (5,16,17). If the LN characteristics are “indeterminate”, further examination is necessary to characterize the LN and perform the appropriate extent of surgery.
Our institution performs elective ipsilateral CCND with frozen analyses in patients with unilateral cN0 PTC to remove occult LN metastasis and accomplish accurate staging.
According to this study, our individual nodal criteria, with a 5-mm cut-off diameter of any metastatic LN, yielded completion TT in 16.2% of patients. If elective CCND with frozen analyses was not performed, it would be difficult to recognize occult metastasis in the central LN, and secondary surgery for completion TT would be inevitable.
Further analyses revealed a significant difference in all nodal characteristics between the LTT and TT groups. Only the size criterion of 5 mm yielded the difference in the number and size of metastatic LNs, LNR, and ENE, which have been independently recognized as significant prognostic factors of PTC (18-21). We believe that metastatic LNs with a diameter of 5 mm may impact other nodal characteristics and can lead to overall nodal aggressiveness.
The current size and number criteria of LN in thyroid cancer in many guidelines have been based on increasing evidence that “metastatic volume” plays a role in prognosis. However, there is no definite consensus on the size and threshold number, resulting in a poor prognosis. Previous studies were mostly retrospective, based on a single institution, with heterogeneous inclusion criteria. In fact, the NCCN guideline version 2020 suggested TT in >5 LN metastasis and >5 mm of the metastatic deposit. The current version showed a reduced size threshold of metastatic LNs to 2 mm. We doubt that sizes 3 and 4 mm of metastatic LNs should be considered more “risky”, because they can also be included in small-volume metastasis. Based on the concept of small-volume LN metastasis, we assume that the size and number criteria of metastatic LNs are relatively uniform and rigid.
Moreover, a decreased size threshold of 2 mm would induce more frequent TT cases. When we classified our patients based on the current version of the NCCN guidelines, TT was indicated in 24.8% of the patients. More patients were involved in TT than in our nodal criteria. This can lead to increased surgical morbidity and thyroid hormone replacement.
An analysis of our study raises the question of whether a 2-mm size and 5 LN threshold are always valuable in all metastatic PTC cases. Further evaluation is needed to better understand the relevant definition and prognostic implications of small-volume LN metastasis in PTC.
Comparative analyses between the LTT and TT groups revealed that the tumor size was also significantly larger in the TT group. Tumor size is one of the most important characteristics of various tumor staging systems. Previous studies have correlated tumor size and LN metastasis in PTC and demonstrated that the larger the tumor, the higher the probability of LN metastasis (22-24). The cut-off varied from 5 to 7 mm. In this study, the TT group has a mean diameter of 1.4±0.9 cm compared with 0.8±0.5 cm in the LTT group. Larger tumor size and more LN metastasis are concordant with the findings of previous studies.
Previous studies have confirmed the association between male sex and LN metastasis in PMC. A majority of studies have demonstrated that male sex is an independent predictor of central LN metastasis in PMC patients, but the impact on prognosis is relatively controversial (24-27). Our analyses showed similar findings that male patients tend to have aggressive LN metastasis, which can lead to TT.
Regarding the analyses of patients according to the NCCN guidelines, younger patients required more completion TT. Age is another significant factor in PTC staging systems. Previous studies have verified that young patients have more LN metastases than older patients. Regarding the relationship with LN metastasis, a cut-off of 55 years was more valuable than 45 years (28,29), which was applied in the eighth edition of the AJCC staging system. In our study, even though the mean ages of both groups were younger than 55 years, younger patients were found to have more metastatic LNs, which is consistent with previous studies.
In clinical practice, after resection of the major central compartment LNs, additional LNs can be identified. Additional LNs can be commonly recognized at the perithyroidal and delphian regions or occasionally at neck level 7. In this study, additional resection resulted in more metastatic LNs in 25 patients (8.6% out of 290 patients, with a mean number of 2.2±1.5 LNs. Eight (2.8%) patients turned from N0 into the N1a stage. These patients had small-volume metastases, and none of them underwent completion TT. Two (0.7%) patients with converted LN results and N stage also had LN micrometastases, which were identified only in the permanent analysis. Cases with converted N stage were relatively rare (3.5%) with small-volume metastasis, and close observation was planned for these patients based on the indolent feature of small-volume metastasis in PTC.
Clinically, the critical case would be the 5 patients who had LTT with larger metastatic LNs (≥5 mm) diagnosed after permanent analyses. However, all metastatic LNs were ultimately removed, and PTC is well-known for a good prognosis after surgical resection (30). Thus, we also determined the close follow-up for these patients.
Even with a few cases of discrepant results, the current diagnostic agreement rate between the frozen analyses and the permanent pathologic report was very high in our institution. This makes intraoperative frozen analysis a highly accurate procedure that can lead to a reliable operation with proper surgical extent, without secondary surgery. Constant effort to increase the sensitivity and NPV of frozen analyses is a crucial future challenge to diminish the discrepant frozen and permanent results.
This study had several limitations. First, this was a retrospective study in a single institution, including a certain time-period surgery with short-term follow-up. We acknowledge that the long-term outcomes of larger subjects are essential to evaluate the relevance of surgical treatments. Therefore, the long-term outcomes of patients with respect to local recurrence or distant metastasis should be consistently investigated. Second, this study was based on our individual criteria for metastatic LN. Third, based on previous studies, the causal relationship between LN metastasis, tumor size, sex, and age was not investigated. Fourth, the other patient and tumor factors that would significantly impact disease prognoses, such as age, sex, tumor size, tumor multiplicity, etc., were not considered in determining surgical extent. Fifth, assessing the cost-effectiveness of the frozen analyses was not feasible to evaluate in this study setting.
Conclusions
In summary, elective CCND with intraoperative frozen analyses is a highly reliable procedure that provides an opportunity to detect occult LN metastasis in patients with cN0 unilateral PTC. Our criteria for metastatic central LN with a cut-off size of 5 mm yielded significant differences in the number and size of the metastatic LN, the LNR, and ENE between the LTT and TT groups, while yielding less completion TT compared with the NCCN guidelines. The concept and clinical significance of small-volume LN metastasis in determining the surgical extent of PTC should be further investigated in larger cohorts with long-term follow-ups.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-15/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-15/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of CHA Ilsan Medical Center (2021-11-005), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784-91. [Crossref] [PubMed]
- Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol 2018;14:670-83. [Crossref] [PubMed]
- Enyioha C, Roman SA, Sosa JA. Central lymph node dissection in patients with papillary thyroid cancer: a population level analysis of 14,257 cases. Am J Surg 2013;205:655-61. [Crossref] [PubMed]
- Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer 2006;106:524-31. [Crossref] [PubMed]
- Ahn JE, Lee JH, Yi JS, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg 2008;32:1552-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. (NCCN Guidelines) Thyroid Carcinoma Version 3.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (Accessed October 19 2021).
- Chan AC, Lang BH, Wong KP. The pros and cons of routine central compartment neck dissection for clinically nodal negative (cN0) papillary thyroid cancer. Gland Surg 2013;2:186-95. [PubMed]
- Kim MJ, Kim CS, Kim JR, et al. Efficiency of intraoperative frozen section analysis of central neck lymph node dissection in patients with papillary thyroid carcinoma. IJS Oncology 2018;3:e67. [Crossref]
- Simpson WJ, McKinney SE, Carruthers JS, et al. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med 1987;83:479-88. [Crossref] [PubMed]
- Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg 1994;18:559-67; discussion 567-8. [Crossref] [PubMed]
- Beasley NJ, Lee J, Eski S, et al. Impact of nodal metastases on prognosis in patients with well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg 2002;128:825-8. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. The Effectiveness of Prophylactic Modified Neck Dissection for Reducing the Development of Lymph Node Recurrence of Papillary Thyroid Carcinoma. World J Surg 2017;41:2283-9. [Crossref] [PubMed]
- Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol 2016;12:981-94. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Tuttle RM, Alzahrani AS. Risk Stratification in Differentiated Thyroid Cancer: From Detection to Final Follow-up. J Clin Endocrinol Metab 2019;104:4087-100. [Crossref] [PubMed]
- Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003;134:946-54; discussion 954-5. [Crossref] [PubMed]
- Kim E, Park JS, Son KR, et al. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008;18:411-8. [Crossref] [PubMed]
- Lango M, Flieder D, Arrangoiz R, et al. Extranodal extension of metastatic papillary thyroid carcinoma: correlation with biochemical endpoints, nodal persistence, and systemic disease progression. Thyroid 2013;23:1099-105. [Crossref] [PubMed]
- Lee SG, Ho J, Choi JB, et al. Optimal Cut-Off Values of Lymph Node Ratio Predicting Recurrence in Papillary Thyroid Cancer. Medicine (Baltimore) 2016;95:e2692. [Crossref] [PubMed]
- Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol 2013;20:1906-11. [Crossref] [PubMed]
- Wang LY, Palmer FL, Nixon IJ, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid 2014;24:1790-5. [Crossref] [PubMed]
- Qu N, Zhang L, Ji QH, et al. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J Surg 2015;39:2459-70. [Crossref] [PubMed]
- Lee KJ, Cho YJ, Kim SJ, et al. Analysis of the clinicopathologic features of papillary thyroid microcarcinoma based on 7-mm tumor size. World J Surg 2011;35:318-23. [Crossref] [PubMed]
- Zheng X, Peng C, Gao M, et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1,587 patients. Cancer Biol Med 2019;16:121-30. [Crossref] [PubMed]
- Tao Y, Wang C, Li L, et al. Clinicopathological features for predicting central and lateral lymph node metastasis in papillary thyroid microcarcinoma: Analysis of 66 cases that underwent central and lateral lymph node dissection. Mol Clin Oncol 2017;6:49-55. [Crossref] [PubMed]
- Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol 2010;6:1771-9. [Crossref] [PubMed]
- Lee YH, Lee YM, Sung TY, et al. Is Male Gender a Prognostic Factor for Papillary Thyroid Microcarcinoma? Ann Surg Oncol 2017;24:1958-64. [Crossref] [PubMed]
- Nixon IJ, Kuk D, Wreesmann V, et al. Defining a Valid Age Cutoff in Staging of Well-Differentiated Thyroid Cancer. Ann Surg Oncol 2016;23:410-5. [Crossref] [PubMed]
- Nixon IJ, Wang LY, Migliacci JC, et al. An International Multi-Institutional Validation of Age 55 Years as a Cutoff for Risk Stratification in the AJCC/UICC Staging System for Well-Differentiated Thyroid Cancer. Thyroid 2016;26:373-80. [Crossref] [PubMed]
- Ulisse S, Baldini E, Lauro A, et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers (Basel) 2021;13:5567. [Crossref] [PubMed]


