Autoimmune pancreatitis
Introduction
Sarles et al. in 1961, first described a disease characterized by chronic inflammatory sclerosis of the pancreas (1). Subsequently in 1995, Yoshida et al. proposed the concept of autoimmune pancreatitis (AIP)—a distinct form of pancreatic disease which had a good response to steroid therapy (2). Further contributions to the understanding of AIP were made in 2001, with the identification of high serum concentration of immunoglobulins G4 (IgG4), which could serve as a biomarker of the disease (3). In recent years, the concept of AIP as a unique clinical entity has gained widespread recognition.
AIP has been described by various terms. These include chronic inflammatory sclerosis of the pancreas (1), chronic sclerosing pancreatitis (4), non-alcoholic duct-destructive chronic pancreatitis (5), lymphoplasmacytic sclerosing pancreatitis (LPSP) (6), idiopathic tumefactive chronic pancreatitis (7) and idiopathic duct-centric chronic pancreatitis (8). AIP can be defined as a distinct form of pancreatitis which is characterized by elevated levels of serum immunoglobulins (notably IgG4), prominent multi-organ infiltration by IgG-positive plasma cells, enlargement of the pancreas, intense fibrotic changes and dramatic response to steroid therapy (9,10).
This review is an update on the current concept of AIP, its clinical presentation, findings on imaging, histopathological features, diagnosis and management.
Classification of AIP
The international consensus diagnostic criteria (ICDC) for AIP recently described two subtypes: type 1 LPSP and type 2 [idiopathic duct-centric pancreatitis (IDCP) or AIP with granulocytic epithelial lesion (GEL)] (9).
Type 1 AIP (LPSP)
Type 1 AIP is the more common form of the disease worldwide, typically prevalent in Japan and Korea (11). References to AIP in the Japanese literature usually refer to type 1 AIP (12). LPSP is the typical histopathological description of type 1 AIP. It is characterized by massive infiltration of lymphoplasmacytic cells without granulocytes; abundant (>10 cells/HPF) IgG4-positive plasma cells; storiform or swirling fibrosis and peri-venular infiltration with lymphoplasmacytic cells which often leads to obliterative phlebitis (8,12,13).
Current understanding of type 1 AIP suggests that it is a pancreatic manifestation of immunoglobulin G4-related disease (IgG4-RD) which is a systemic inflammatory disorder of unknown cause (14-16). Extra-pancreatic organ involvement is common in type 1 AIP. It is important to note that serum levels of IgG4 are known to fluctuate (17); a subset of type 1 AIP patients do not have the typically elevated serum levels of IgG4 and that seronegativity in itself should not be used to reclassify such patients as type 2 AIP (14).
Type 2 AIP (IDCP, GEL)
The histopathological pattern observed in type 2 AIP has been described as IDCP or AIP with GEL. It is characterized by prominent infiltration of the epithelium and or lumen of the interlobular pancreatic ducts by neutrophils which may lead to the destruction and obliteration of the pancreatic duct.
Notably, type 2 AIP in contrast to type 1 AIP, is a pancreas-specific disease mostly without extra-pancreatic organ involvement, lacks elevated serum levels of IgG4 and auto antibodies. Type 2 AIP is associated with inflammatory bowel disease (approximately 30%) and demonstrates little or no IgG4-positive inflammatory infiltrates on histology (12,18). Histopathological review is required to confirm the diagnosis of type 2 AIP because of the lack of serological markers and specific imaging patterns.
Epidemiology
AIP is a rare disease with an overall prevalence rate of 2.2 per 100,000 populations and a reported annual incidence rate of 0.9 per 100,000 populations in Japan (19). Type 1 AIP as earlier alluded to, is the most prevalent subtype worldwide. In the US, it accounts for more than 80% of the cases. Type 2 AIP however, is relatively more common in Europe although type 1 AIP still remains the more prevalent subtype (20). Type 1 AIP often presents at an older age. An international multicenter survey observed that type 1 AIP patients were approximately 16 years older than patients with type 2 AIP (11). A Japanese national survey reported a mean age of 63 years for patients with AIP (19).
In contrast to the observed male predilection in type 1 AIP; there is no gender predilection in type 2 AIP (18). This is consistent with the findings of a Japanese national survey which reported a male to female ratio of 3.7:1 (19), type 1 AIP being the most prevalent form in Japan. A systematic review of AIP in China reported a male to female ratio of 4.5:1 and type 2 AIP accounted for 4.7% of the patients in the review (21).
Pathogenesis
The pathogenesis of AIP is unknown; however it appears that autoimmune processes play a significant role. Evidence suggestive of the role of immunologic mechanisms include the presence of elevated levels of autoantibodies, characteristic lymphoplasmacytic infiltration on histology, hypergammaglobulinemia and a predictable response to steroids (22,23).
Human leucocyte antigen (HLA) investigation studies in the Japanese population suggests a possible association between DRB1*0405-DQB1*0401 haplotype and AIP (24). This association was however not confirmed by a similar study carried out in the South Korean population. The South Korean study affirms that the substitution of aspartic acid to non-aspartic acid at DQB1 57 appears to represent a key genetic factor for the relapse of AIP (25).
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) which is expressed on CD4+ and CD8+ T cells is an important regulator of T cell stimulation. It suppresses T cell proliferation and inflammatory cytokine production, sets the threshold for T cell activation and induces apoptosis in activated T cells. Investigations into the underlying pathogenesis of AIP in Japanese and Chinese patients suggest an association between genetic single nucleotide polymorphism in CTLA-4 and AIP (26,27). Further studies of the role of genetics in the aetiopathogenesis of AIP are still being awaited.
Multiple disease-related antibodies have been associated with AIP. These include anti-heat shock protein (HSP) 10 (28), anti-amylase alpha (29), anti-carbonic anhydrase II, anti-lactoferrin (30), anti-carbonic anhydrase IV (31), anti-plasminogen binding protein (anti-PBP) and anti-pancreatic secretory trypsin inhibitor (32). Evidence in support of the associations and the possible role of these antibodies in AIP, is the observed induction of systemic lesions similar to human AIP such as pancreatitis, interstitial nephritis and sialadenitis, following intradermal immunization with carbonic anhydrase II and lactoferrin in animal models. The human AIP-like systemic lesions were characterized by significant increase in the number and the size of foci of lymphocytic infiltration (33).
A plausible theory in the pathogenesis of AIP includes the potential role of molecular mimicry. There is a significant homology between human carbonic anhydrase II and alpha-carbonic anhydrase of Helicobacter pylori. The homologous segments contain the binding motif of the HLA molecule DRB1*0405 which in theory suggests that H. pylori infection could possibly trigger AIP via molecular mimicry in genetically predisposed individuals (34). Other plausible hypotheses that could benefit from further investigations include the role of the complement activation system via the classic pathway (35), Th2 cells and regulatory T cells in AIP (Treg) (36).
Clinical presentation
The clinical findings in AIP are non-specific and the most common presentation is obstructive jaundice. A recent systematic review of AIP in China showed that obstructive jaundice accounted for 63.4% of the 706 patients in the study (21). This is fairly consistent with earlier findings of a multicenter survey of 731 patients, in which obstructive jaundice accounted for 75% and 47% of the patients with type 1 and type 2 AIP respectively (11).
Patients with type 2 AIP may present with severe abdominal pain (68% vs. 41%) in contrast to type 1 AIP patients. The abdominal pain in type 1 AIP is described as mild, not as severe as the abdominal pain observed in acute pancreatitis or acute exacerbation of chronic pancreatitis (9,11,12). Patients can also present with symptoms of diabetes mellitus or symptoms consistent with extra pancreatic associations seen in AIP. Other symptoms include back pain, weight loss and fatigue (12,16).
Serology
IgG4 comprises 4-6% of the total IgG in healthy individuals and raised serum levels occurs rarely in certain conditions such as allergic diseases, parasitic infestations and pemphigus vulgaris (37). Rare serum elevations of IgG4 occur in 5% of the normal population and 10% of patients with pancreatic cancer (17). Although not disease-specific as highlighted above, IgG4 has the highest diagnostic value as a single serological test in AIP. AIP is associated with elevated serum levels of IgG4. A cut-off value for serum IgG4 concentrations of 135 mg/dL with 97% accuracy, 95% sensitivity and 97% specificity for differentiating AIP from pancreatic cancer has been reported (3). A subsequent study reported 76% sensitivity, 93% specificity and 36% positive predictive value for elevated serum IgG4 (>140 mg/dL) in AIP (38).
Other serological findings in AIP include hypergammaglobulinemia, elevated levels of IgG, antinuclear antibodies, anti-smooth muscle antibodies, carbonic anhydrase II antibodies, lactoferrin antibodies and rheumatoid factor (12,30).
Extrapancreatic associations
AIP is associated with extrapancreatic lesions and the biliary tree is the most common extrapancreatic site involved in AIP (39). Type 1 AIP is currently viewed as a pancreatic manifestation of IgG4-RD because of its association with a variety of extrapancreatic lesions. A study of 100 patients with AIP in China reported the occurrence of extrapancreatic lesions in 77% of the patients (16).
Evidence in support of the association between other organ involvement and AIP includes (I) shared characteristic histopathological findings of lymphoplasmacytic infiltration, IgG4-positive plasma cell infiltration, obliterative phlebitis and storiform fibrosis; (II) frequent co-existence or occurrence; (III) predictably favorable response to steroids and (IV) differentiation from the lesions of the corresponding organs such as distinction between AIP-associated salivary gland lesions and lesions due to Sjogren’s syndrome (37). These lesions include sialadenitis (40), sclerosing cholangitis (41), retroperitoneal fibrosis (42), interstitial lung disease (43) and tubulointerstitial nephritis (44).
Imaging
Imaging plays an important role in the diagnostic work up for AIP as reflected in the different existing diagnostic criteria. AIP demonstrates a wide spectrum of imaging findings on CT. Although diffuse morphological pancreatic parenchymal enlargement is seen in 40–60% of patients with AIP (45-48), three other morphological patterns have been described. These include focal enlargement of the pancreas; no enlargement or normal pancreas in a minority of the patients; and mixed patterns (21,47-49). Typically, AIP demonstrates a diminished pattern of enhancement in the early or arterial phase and a relatively increased or prolonged enhancement in the delayed or venous phase (46). A capsule-like low density rim is a distinctive finding on CT in AIP. The cord or band-like structure that surrounds the lesion demonstrates lower absorption than the pancreatic parenchymal during the parenchymal phase and delayed enhancement pattern on dynamic imaging studies. This gives rise to the capsule or the rim sign of AIP (12,46,48,50). It must be emphasized that the absence of the typical findings of AIP on CT is not enough reason to rule out AIP as a differential diagnosis.
Typical MRI findings in AIP include hypo-intense signal on T1 weighted images, lower signal intensity in the presence of intense fibrosis or relatively T2 hyper intensity with minimal fibrosis (46,51). MRI also demonstrates the typical capsule-like rim as a hypo-intense rim on both T1 and T2 weighted images and shows a delayed enhancement on dynamic MR study (46).
Varying findings in AIP on EUS have been reported. These include diffuse enlargement, hypoechoic pancreas or a focal hypoechoic mass (52). Other findings include hyperechoic foci in the pancreatic parenchymal, hyperechoic strands, lobularity and lobular outer margins (53). Although EUS has a high local resolution, its use in the diagnosis of AIP has been largely restricted to EUS-guided fine needle aspiration (EUS-FNA) or EUS-guided trucut biopsy aimed at obtaining histological evidence for AIP (54). The reason for this may be the varying EUS findings reported by authors and the non-specific nature of these findings.
Consistent with the diagnostic criteria for AIP, narrowing of the main pancreatic duct (MPD) on endoscopic retrograde pancreatography (ERCP) is characteristic. The sensitivity and specificity of ERCP in the diagnosis of AIP is 71% and 83% respectively (55). An international study highlighted four important features that were highly suggestive of AIP on ERCP; long (>1/3 the length of the pancreatic duct) stricture; lack of upstream dilatation from the stricture (<5 mm); multiple strictures; and side branches arising from a segment with stricture (55). Abnormal findings in the biliary system (both intra-hepatic and extrahepatic bile duct) are common in AIP. About 80–90% of patients with AIP demonstrate narrowing of the lower common bile duct with varying degree of stenosis (56).
Diagnostic criteria
Given the protean clinical features of AIP and the need for accurate diagnosis, various diagnostic criteria have evolved over the years from different research and clinical groups. The Japan Pancreas Society (JPS) in 2002 proposed a diagnostic criteria for AIP and subsequently revised it in 2006 and 2011 (12,57). The Korean criteria for AIP was proposed by Asan Medical Centre of Korea in 2006. A consensus between the Korean Society of Pancreatobiliary Diseases and the Japanese Research Committee of Intractable Pancreatic Diseases subsequently led to the Asian criteria (57).
The Mayo clinic HISORt criteria for AIP is based on five cardinal features which include typical findings on histology, imaging, serology, other organ involvement and response to steroid therapy (58).
The JPS 2011 diagnostic criteria include; appearance of diffuse and segmental/focal type in pancreatic parenchymal CT/MRI images or ERCP duct images; a single category without level 1 and 2 classifications in the ICDC; IgG4 alone as a serum marker; histopathological criteria for LPSP; sclerosing cholangitis, sclerosing sialadenitis and retroperitoneal fibrosis as typical other organ involvement; and response to an optional steroid trial after using EUS-FNA to rule out malignancy (12). Following a review of the existing diagnostic criteria, the ICDC for AIP (ICDC-2011) differentiated the two subtypes of AIP with level 1 and 2 classifications (Tables 1-4).
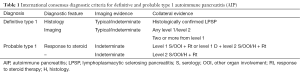
Full table

Full table

Full table
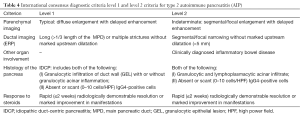
Full table
Differential diagnosis
Given the clinical and imaging features of AIP, the most concerning differential diagnosis of AIP is cancer of the pancreas. A study of 100 patients with AIP reported that 28% of the patients had initial surgical procedures such as pancreatoduodenectomy, distal pancreatectomy with or without splenectomy etc. for suspected pancreatic malignancy (16). Consistent with this report is the findings of a systematic review of 26 articles with a total of 706 patients with AIP which reported that 29.7% of the patients were misdiagnosed as pancreatic cancer and the patients had surgical intervention (21).
Strategies have been published with the aim of differentiating AIP from pancreatic cancer. A Japanese strategy evolved from a comparative study of the clinical, serological and imaging features of 17 patients who presented with a focal enlargement (mass-like lesion) of the head of the pancreas and 70 patients with cancer of the head of the pancreas. The study emphasized that clinical features were not enough to distinguish AIP and cancer of the head of the pancreas. The study highlighted six imaging (CT and ERCP) characteristics that were highly suggestive of AIP. These features include: delayed enhancement of an enlarged pancreas (CT); a capsule-like rim around the pancreas (CT); extrapancreatic lesions such as salivary gland involvement, retroperitoneal mass, or stenosis of the intrahepatic bile duct (CT/ERCP); MPD narrowing ≥3 cm long (ERCP); skipped lesions (multiple areas of narrowing) of the MPD (ERCP); and maximal diameter of the upstream MPD dilation ≤5 mm above the stricture (ERCP) (59).
The second strategy with its algorithm was proposed by the Mayo clinic in the US. The differences between both strategies are the inclusion of all imaging subtypes of AIP in the Mayo clinic strategy in contrast to the Japanese strategy which focused on AIP with mass like lesions on imaging (60). The Japanese strategy also relied on ERCP and this probably reflects the differences in the clinical practice in both countries.
Treatment
The mainstay of therapy for AIP is steroids. A large multicenter (23 institutions) study involving 1,064 patients with AIP based on the ICDC classification (type 1 n=978; type 2 n=86) from 10 different countries reported that most of the patients with type 1 (99%) and type 2 (92%) AIP who were treated with steroids went into clinical remission (61). This is consistent with an earlier retrospective survey of 563 patients in 17 centers in Japan which reported a remission rate of 98% for patients with AIP who were treated with steroids (62).
There is evidence to suggest that some patients may have spontaneous resolution of AIP which includes reduction in the swelling of the pancreas (27%) (63) and spontaneous improvement (9%) in non-jaundiced patients (64). The indications for steroid therapy in AIP patients include jaundice, abdominal pain, abnormal imaging and other organ involvement (retroperitoneal fibrosis, salivary gland enlargement, IgG4-related renal disease, lymphadenopathy, inflammatory bowel disease etc.) (61,65).
The majority of patients with jaundice required biliary stent placement (71% of type 1 and 77% of type 2 AIP). Relapses were more common in patients with type 1 (31%) vs. type 2 AIP (9%), especially those with IgG4-related sclerosing cholangitis (56% vs. 26%) and the relapses commonly occurred in the pancreas and/or biliary system. Restarting treatment with steroids effectively induced remission with or without alternative treatment, such as azathioprine (61).
In conclusion, AIP is a rare and distinct form of pancreatitis which has been classified into two subtypes; type 1 and type 2. The diagnostic criteria requires a variable combination of histopathological, imaging and serological features in the presence of typical extrapancreatic lesions and a predictable response to steroid therapy. AIP is a steroid-responsive disease which should be considered as a differential diagnosis in the evaluation of pancreatic diseases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sarles H, Sarles JC, Muratore R, et al. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis 1961;6:688-98. [Crossref] [PubMed]
- Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 1995;40:1561-8. [Crossref] [PubMed]
- Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732-8. [Crossref] [PubMed]
- Sood S, Fossard DP, Shorrock K. Chronic sclerosing pancreatitis in Sjögren's syndrome: a case report. Pancreas 1995;10:419-21. [Crossref] [PubMed]
- Ectors N, Maillet B, Aerts R, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut 1997;41:263-8. [Crossref] [PubMed]
- Weber SM, Cubukcu-Dimopulo O, Palesty JA, et al. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg 2003;7:129-37; discussion 137-9. [Crossref] [PubMed]
- Yadav D, Notahara K, Smyrk TC, et al. Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clin Gastroenterol Hepatol 2003;1:129-35. [Crossref] [PubMed]
- Notohara K, Burgart LJ, Yadav D, et al. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol 2003;27:1119-27. [Crossref] [PubMed]
- Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 2011;40:352-8. [Crossref] [PubMed]
- Ito T, Nishimori I, Inoue N, et al. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol 2007;42 Suppl 18:50-8. [Crossref] [PubMed]
- Kamisawa T, Chari ST, Giday SA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 2011;40:809-14. [Crossref] [PubMed]
- Okazaki K, Kawa S, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 2014;49:567-88. [Crossref] [PubMed]
- Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 2006;41:613-25. [Crossref] [PubMed]
- Sah RP, Chari ST. Autoimmune pancreatitis: an update on classification, diagnosis, natural history and management. Curr Gastroenterol Rep 2012;14:95-105. [Crossref] [PubMed]
- Lee LK, Sahani DV. Autoimmune pancreatitis in the context of IgG4-related disease: review of imaging findings. World J Gastroenterol 2014;20:15177-89. [Crossref] [PubMed]
- Xin L, He YX, Zhu XF, et al. Diagnosis and treatment of autoimmune pancreatitis: experience with 100 patients. Hepatobiliary Pancreat Dis Int 2014;13:642-8. [Crossref] [PubMed]
- Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 2011;23:108-13. [Crossref] [PubMed]
- Zamboni G, Lüttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch 2004;445:552-63. [Crossref] [PubMed]
- Kanno A, Nishimori I, Masamune A, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas 2012;41:835-9. [Crossref] [PubMed]
- Sugumar A, Klöppel G, Chari ST. Autoimmune pancreatitis: pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol 2009;104:2308-10. [Crossref] [PubMed]
- Meng Q, Xin L, Liu W, et al. Diagnosis and Treatment of Autoimmune Pancreatitis in China: A Systematic Review. PLoS One 2015;10:e0130466. [Crossref] [PubMed]
- Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep 2005;7:101-6. [Crossref] [PubMed]
- Sugumar A, Takahashi N, Chari ST. Distinguishing pancreatic cancer from autoimmune pancreatitis. Curr Gastroenterol Rep 2010;12:91-7. [Crossref] [PubMed]
- Kawa S, Ota M, Yoshizawa K, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology 2002;122:1264-9. [Crossref] [PubMed]
- Park do H, Kim MH, Oh HB, et al. Substitution of aspartic acid at position 57 of the DQbeta1 affects relapse of autoimmune pancreatitis. Gastroenterology 2008;134:440-6. [Crossref] [PubMed]
- Umemura T, Ota M, Hamano H, et al. Association of autoimmune pancreatitis with cytotoxic T-lymphocyte antigen 4 gene polymorphisms in Japanese patients. Am J Gastroenterol 2008;103:588-94. [Crossref] [PubMed]
- Chang MC, Chang YT, Tien YW, et al. T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem 2007;53:1700-5. [Crossref] [PubMed]
- Takizawa S, Endo T, Wanjia X, et al. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem Biophys Res Commun 2009;386:192-6. [Crossref] [PubMed]
- Endo T, Takizawa S, Tanaka S, et al. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 2009;58:732-7. [Crossref] [PubMed]
- Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000;118:573-81. [Crossref] [PubMed]
- Nishimori I, Miyaji E, Morimoto K, et al. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 2005;54:274-81. [Crossref] [PubMed]
- Asada M, Nishio A, Uchida K, et al. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 2006;33:20-6. [Crossref] [PubMed]
- Nishimori I, Bratanova T, Toshkov I, et al. Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol 1995;154:4865-73. [PubMed]
- Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med 2005;9:741-4. [Crossref] [PubMed]
- Muraki T, Hamano H, Ochi Y, et al. Autoimmune pancreatitis and complement activation system. Pancreas 2006;32:16-21. [Crossref] [PubMed]
- Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45:1538-46. [Crossref] [PubMed]
- Kawa S, Okazaki K, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 II. Extrapancreatic lesions, differential diagnosis. J Gastroenterol 2014;49:765-84. [Crossref] [PubMed]
- Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007;102:1646-53. [Crossref] [PubMed]
- Ketwaroo GA, Sheth S. Autoimmune pancreatitis. Gastroenterol Rep (Oxf) 2013;1:27-32. [Crossref] [PubMed]
- Kamisawa T, Funata N, Hayashi Y, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut 2003;52:683-7. [Crossref] [PubMed]
- Nakazawa T, Ohara H, Yamada T, et al. Atypical primary sclerosing cholangitis cases associated with unusual pancreatitis. Hepatogastroenterology 2001;48:625-30. [PubMed]
- Hamano H, Kawa S, Ochi Y, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet 2002;359:1403-4. [Crossref] [PubMed]
- Hirano K, Kawabe T, Komatsu Y, et al. High-rate pulmonary involvement in autoimmune pancreatitis. Intern Med J 2006;36:58-61. [Crossref] [PubMed]
- Uchiyama-Tanaka Y, Mori Y, Kimura T, et al. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis 2004;43:e18-25. [Crossref] [PubMed]
- Bodily KD, Takahashi N, Fletcher JG, et al. Autoimmune pancreatitis: pancreatic and extrapancreatic imaging findings. AJR Am J Roentgenol 2009;192:431-7. [Crossref] [PubMed]
- Irie H, Honda H, Baba S, et al. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol 1998;170:1323-7. [Crossref] [PubMed]
- Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features. Radiology 2004;233:345-52. [Crossref] [PubMed]
- Takahashi N, Fletcher JG, Fidler JL, et al. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol 2008;190:280-6. [Crossref] [PubMed]
- Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4:1010-6. [Crossref] [PubMed]
- Yang DH, Kim KW, Kim TK, et al. Autoimmune pancreatitis: radiologic findings in 20 patients. Abdom Imaging 2006;31:94-102. [Crossref] [PubMed]
- Rehnitz C, Klauss M, Singer R, et al. Morphologic patterns of autoimmune pancreatitis in CT and MRI. Pancreatology 2011;11:240-51. [Crossref] [PubMed]
- Farrell JJ, Garber J, Sahani D, et al. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc 2004;60:927-36. [Crossref] [PubMed]
- Okabe Y, Ishida Y, Kaji R, et al. Endoscopic ultrasonographic study of autoimmune pancreatitis and the effect of steroid therapy. J Hepatobiliary Pancreat Sci 2012;19:266-73. [Crossref] [PubMed]
- Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc 2005;61:467-72. [Crossref] [PubMed]
- Sugumar A, Levy MJ, Kamisawa T, et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: an international multicentre study. Gut 2011;60:666-70. [Crossref] [PubMed]
- Horiuchi A, Kawa S, Hamano H, et al. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc 2002;55:494-9. [Crossref] [PubMed]
- Otsuki M, Chung JB, Okazaki K, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 2008;43:403-8. [Crossref] [PubMed]
- Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol 2007;42 Suppl 18:39-41. [Crossref] [PubMed]
- Kamisawa T, Imai M, Yui Chen P, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas 2008;37:e62-7. [Crossref] [PubMed]
- Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol 2009;7:1097-103. [Crossref] [PubMed]
- Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771-6. [Crossref] [PubMed]
- Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009;58:1504-7. [Crossref] [PubMed]
- Wakabayashi T, Kawaura Y, Satomura Y, et al. Long-term prognosis of duct-narrowing chronic pancreatitis: strategy for steroid treatment. Pancreas 2005;30:31-9. [PubMed]
- Kamisawa T, Yoshiike M, Egawa N, et al. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology 2005;5:234-8; discussion 238-40. [Crossref] [PubMed]
- Kamisawa T, Okazaki K, Kawa S, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol 2014;49:961-70. [Crossref] [PubMed]


