
Impact of the tumor immune microenvironment on the outcome of pancreatic cancer: a retrospective study based on clinical pathological analysis
Introduction
Pancreatic cancer (PC) is one of the most aggressive tumors of the digestive system, with insidious and atypical clinical symptoms, rapid progression, and extremely high malignancy. The 5-year overall survival rate after diagnosis is less than 5% (1). Surgery is still the first choice for the treatment of PC, but residual tumor cells in the operation field and subclinical metastasis in the non-surgical area are likely to cause postoperative recurrence, and patients are not sensitive to radiotherapy and chemotherapy. Besides, the 5-year overall survival rate after surgery rarely exceeds 10–19%, so it is necessary to find new diagnosis and treatment methods (2).
Immunotherapy, following the radiotherapy and chemotherapy, represents another important anti-tumor method after surgery. It activates the immune system and kills cancer cells and tumor tissues by relying on autoimmune functions. In recent years, immunotherapy has achieved remarkable therapeutic effects in solid tumors such as melanoma and lung cancer (3). However, immunotherapy does not provide obvious benefit for PC when used as a single agent mainly due to the intrinsic low immunogenicity and the establishment of a powerful immunosuppressive tumor microenvironment (4). The formation of immunosuppressive microenvironment caused an escape of tumor cells from immune surveillance, limiting the activation and function of immune cells, eventually leading to an unimproved poor prognosis with a 5-year survival less than 8% (5). In pancreatic cancer, the immunosuppressive microenvironment consists of tumor-associated macrophages, myeloid-derived suppressor cells, CD4+/CD8+T lymphocytes, regulatory T cells (Treg), regulatory B cells and so on (6). To date, the antitumor immune response is known to be downregulated in the complicated pancreatic microenvironment, particular for the functionally exhausted T cells. CD4+ T cells play a central role in regulating tumor immune effects. CD4+IL-17+ T cells are a new type of effector CD4+ T cell discovered in recent years, which can promote the development of tumors by inducing angiogenesis and recruiting inflammatory cells (7). Relevant data show that CD4+IL-17+ T cells play a key role in autoimmune diseases and infectious diseases (8). Previous studies uncovered that IL17 recruited neutrophils, and excluded cytotoxic CD8+ T cells from tumors. IL-17 blockade increases immune checkpoint blockade (PD-1, CTLA4) sensitivity (9,10). CD8+ T cells is an important subset in tumor immune surveillance. Initial CD8+ T cells can differentiate into cytotoxic CD8+ T cells in response to the stimulation of cancer cell antigens, which can kill antigen-specific cancer cells and inhibit tumorigenesis and development. Therefore, our focused the expression of CD4, IL-17 and CD8 in PC based on their pivotal roles in regulating immune function and affecting immunotherapy efficiency.
In spite that several studies have indicated the effects of CD4+ T cells, CD8+ T cells and regulatory T cells on prognosis of patients with PC, no consensus has been achieved with regarding to their contributions. Study by Wang et al. revealed that high CD4+ and high CD8+ T cells were correlated with good prognosis compared with low CD4+ and low CD8+ T cells (11). While, Kumai et al. showed that the frequency of CD4+ T cells in pancreatic cancer tissues did not affect prognosis after surgery, but prognosis was good in the group with a high frequency of CD8+ T cells and low frequency of regulatory T cells (12). MacNeil reported that high CD8 significantly predicted improved patient overall survival (13). Therefore, the distribution and function of these cells and their related cytokines in the tumor microenvironment of PC are not well characterized due to their variation across different sites of disease and their effects on malignant cell state and therapeutic response (14). In this study, samples were gathered from 40 patients with PC. The expression of CD4, IL-17, and CD8 were measured by immunohistochemical staining. We then explored the correlation between CD4+IL-17+ T cells and CD8+ T cells and the clinicopathological characteristics and outcomes of patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-45/rc).
Methods
Subject selection
The tumor tissue specimens of 40 patients with PC in the Third Xiangya Hospital from May 2016 to May 2019 were retrospectively analyzed. Normal pancreatic tissues adjacent to the tumor were collected as controls. The patients were 33 to 85 years old, with an average age of 61.22±10.58 years old. There were 21 males and 19 females. The inclusion criteria were as follows: (I) all were diagnosed with PC by postoperative histopathology; (II) tissue specimens were obtained by surgical resection; (III) no radiotherapy, chemotherapy, and immunotherapy were performed before the operation; (IV) no hormones and immunosuppressive agents were previously taken; (V) the medical records and follow-up data were complete; (VI) the patients and their family members signed the informed consent. The exclusion criteria were as follows: (I) patients with autoimmune and infectious diseases; (II) patients with secondary PC; (III) patients with distant metastasis before surgery; (IV) patients with combined portal vein resection or combined resection of metastatic organs; (V) combined with other blood system diseases and solid tumors; (VI) patients with unclear pathological diagnosis after surgery. The flow chart in Figure 1 depicts the patient selection process and reasons for exclusion. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of the Third Xiangya Hospital [No. SYXK(Xiang)2017-0002] and informed consent was taken from all the patients.
Immunohistochemistry (IHC) staining
To determine the infiltrated CD4+, IL-17+ and CD8+ T cells in PC cancer and adjacent tissues, IHC analysis was performed. The surgically resected samples were immediately fixed with 10% neutral formaldehyde and routinely embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). The 4 µm thick sections were routinely deparaffinized and hydrated. The sections were first blocked by H2O2 and then incubated with normal serum for 10 minutes, and then incubated with the primary antibody at 4 °C overnight. After rewarming at 37 °C, the secondary antibody was added to sections and incubated for 1 hour. Finally, the tissue sections were color-developed by diaminobenzidine (DAB), stained with hematoxylin, dehydrated, cleared, and sealed. Meanwhile, samples with known positive staining were used as positive controls, and phosphate buffer saline (PBS) was used as the negative control.
Scoring of staining results
Two senior pathologists reviewed and observed the slices by the double-blind method. Ten high power fields were examined in every section, and 100 tumor cells were counted in each high power field. Positive expression of CD4 and CD8 was shown by brownish-yellow particles located in the cell membrane, while positive expression of IL-17 was shown by brown particles located in the cytoplasm. The judgment criteria of staining intensity were as follows: sepia: 3 points, brown: 2 points, faint yellow: 1 point, and colorlessness: 0 point. According to the percentage of stained cells, >75% was 3 points, 51–75% was 2 points, 11–50% was 1 point, and ≤10% was 0 point. The product of the staining intensity score and staining range score was calculated, and 0–2 was negative while more than 3 was positive.
Statistical analysis
Data in this study are represented by . Count data are shown as n (%) and compared using the independent sample t-test. Group comparisons were conducted by the χ2 test. Kaplan-Meier survival curves were plotted for survival analysis. Survival rates were measured by the log-rank test. The Cox proportional risk model was applied to investigate the risk factors influencing the outcome of PC patients, and the nomogram for predicting survival was established. P<0.05 indicates statistical significance. Data were analyzed using the SPSS20.0 software package and R 3.4.1 software.
Results
The expression of CD4, IL-17, and CD8 in PC
The results of immunohistochemical staining showed that CD4 was mainly distributed in the cell membrane in PC tissues, with brownish-yellow particles. There were 32 cases (80.00%) with CD4 positive expression out of the 40 PC tissues, and 18 cases (45.00%) had CD4 positive expression in adjacent tissues. IL-17 was distributed in the cytoplasm of PC tissues as brown granules. IL-17 was positively expressed in 26 of the 40 PC tissues (65.00%) and 15 of the corresponding adjacent tissues (37.50%). CD8 was distributed in the cell membrane in PC tissues, showing brownish-yellow particles. CD8 was expressed in 16 of the 40 PC tissues (40.00%) and 33 of the corresponding adjacent tissues (82.50%). The positive expression of CD4, IL-17, and CD8 in PC and adjacent samples had significant differences (P<0.05, Table 1).
Table 1
| Tissue | CD4, n (%) | IL-17, n (%) | CD8, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||
| PC | 32 (80.00) | 8 (20.00) | 26 (65.00) | 14 (35.00) | 16 (40.00) | 24 (60.00) | ||
| Adjacent | 18 (45.00) | 22 (55.00) | 15 (37.50) | 25 (62.50) | 33 (82.50) | 7 (17.50) | ||
| χ2 | 10.453 | 6.054 | 15.221 | |||||
| P | 0.001 | 0.014 | <0.001 | |||||
PC, pancreatic cancer.
Cell counts of CD4+IL-17+ and CD8+ T cells in PC tissues
The percentage of CD4+IL-17+ T cells in PC samples was significantly higher than that in normal adjacent samples, and the number of CD8+ T cells was markedly lower than that in normal samples, with significant differences (P<0.01, Table 2).
Table 2
| Group | Cases | CD4+IL-17+ | CD8+ |
| PC | 40 | 5.32±1.90 | 24.25±3.05 |
| Adjacent | 40 | 3.35±1.27 | 43.11±4.62 |
| t | 5.452 | 21.546 | |
| P | <0.001 | <0.001 |
PC, pancreatic cancer.
Correlation between the expression of CD4, IL-17, and CD8 and clinicopathological features in PC tissues
The expression level of CD4 in PC tissues was strongly associated with TNM stage and lymph node metastasis (P<0.05). Furthermore, the expression of IL-17 and CD8 were significantly associated with histological grade, TNM stage, lymph node metastasis, and local infiltration (P<0.05, Table 3).
Table 3
| Clinicopathological features | Subtypes | Cases | CD4, n (%) | IL-17, n (%) | CD8, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | P | Positive | Negative | P | Positive | Negative | P | |||||
| Gender | Male | 21 | 17 (80.95) | 4 (19.05) | 0.874 | 14 (66.67) | 7 (33.33) | 0.816 | 9 (42.86) | 12 (57.14) | 0.698 | ||
| Female | 19 | 15 (78.95) | 4 (21.05) | 12 (63.16) | 7 (36.84) | 7 (36.84) | 12 (63.16) | ||||||
| Age (years) | ≤60 | 17 | 14 (82.35) | 3 (17.65) | 0.749 | 11 (64.71) | 6 (35.29) | 0.973 | 8 (47.06) | 9 (52.94) | 0.433 | ||
| >60 | 23 | 18 (78.26) | 5 (21.74) | 15 (65.22) | 8 (34.78) | 8 (34.78) | 15 (65.22) | ||||||
| Tumor size (cm) | ≤2 | 18 | 13 (72.22) | 5 (27.78) | 0.266 | 13 (72.22) | 5 (27.78) | 0.088 | 10 (55.56) | 8 (44.44) | 0.069 | ||
| >2 | 22 | 19 (86.36) | 3 (13.64) | 10 (45.45) | 12 (54.55) | 6 (27.27) | 16 (72.73) | ||||||
| Tumor site | Head of pancreas | 27 | 21 (77.78) | 6 (22.22) | 0.613 | 18 (66.67) | 9 (33.33) | 0.750 | 12 (44.44) | 15 (55.56) | 0.408 | ||
| Body tail | 13 | 11 (84.62) | 2 (15.38) | 8 (61.54) | 5 (38.46) | 4 (30.77) | 9 (69.23) | ||||||
| Histological grade | Poorly differentiated | 9 | 7 (77.78) | 2 (22.22) | 0.395 | 7 (77.78) | 2 (22.22) | 0.006* | 6 (66.67) | 3 (33.33) | 0.047* | ||
| Moderately differentiated | 18 | 16 (88.89) | 2 (11.11) | 7 (38.89) | 11 (61.11) | 8 (44.44) | 10 (55.56) | ||||||
| High differentiated | 13 | 9 (69.23) | 4 (30.77) | 12 (92.31) | 1 (7.69) | 2 (15.38) | 11 (84.62) | ||||||
| TNM stage | I–II stage | 22 | 15 (68.18) | 7 (31.82) | 0.039* | 10 (45.45) | 12 (54.55) | 0.004* | 13 (59.09) | 9 (40.91) | 0.006* | ||
| III–IV stage | 18 | 17 (94.44) | 1 (5.56) | 16 (88.89) | 2 (11.11) | 3 (16.67) | 15 (83.33) | ||||||
| Lymph node metastasis | Yes | 21 | 14 (66.67) | 7 (33.33) | 0.027* | 17 (80.95) | 4 (19.05) | 0.026* | 4 (19.05) | 17 (80.95) | 0.004* | ||
| No | 19 | 18 (84.74) | 1 (5.26) | 9 (47.37) | 10 (52.63) | 12 (63.16) | 7 (36.84) | ||||||
| Local infiltration | Yes | 23 | 18 (78.26) | 5 (21.74) | 0.749 | 20 (86.96) | 3 (13.04) | 0.001* | 5 (21.74) | 18 (78.26) | 0.006* | ||
| No | 17 | 14 (82.35) | 3 (17.65) | 6 (35.29) | 11 (64.71) | 11 (64.71) | 6 (35.29) | ||||||
*, P<0.05 represents significant difference. PC, pancreatic cancer; TNM, tumor node metastasis.
The relationship between the expression of CD4, IL-17, CD8 and the clinicopathological characteristics and prognosis of patients
The 40 patients in this study were followed up for 2–40 months, and the average survival time was 16.85±8.21 months. The median survival times (MSTs) of patients with TNM stages I–II and III–IV were 12.5 and 23.0 months, respectively. The MSTs were 12.3 and 20.8 months for patients who had or did not have lymph node metastasis, respectively. The MSTs were 13.2 and 21.4 months in CD4 positive and negative patients, respectively. The MSTs were 10.4 and 24.8 months in IL-17 positive and negative patients, respectively. The MSTs for CD8 positive and negative patients were 21.9 and 11.8 months, respectively. The differences between groups were statistically significant (P<0.05), as shown in Table 4 and Figure 2.
Table 4
| Factors | P | HR | 95% CI |
|---|---|---|---|
| TNM stage I–II vs. III-IV | 0.004 | 3.276 | 1.578–5.422 |
| Lymph node metastasis (yes vs. no) | 0.003 | 2.318 | 1.458–4.112 |
| CD4 positive vs. negative | <0.001 | 2.359 | 2.116–5.201 |
| IL-17 positive vs. negative | <0.001 | 3.185 | 2.156–5.871 |
| CD8 positive vs. negative | <0.001 | 2.323 | 1.027–6.278 |
HR, hazard ratio; 95% CI, 95% confidence interval; TNM, tumor node metastasis.

Multivariate analysis of factors affecting the prognosis of PC by the Cox regression model
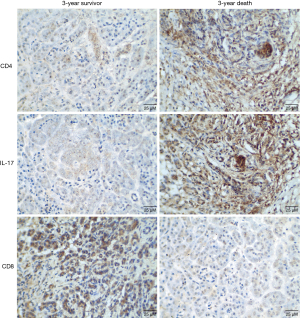
Cox regression model analysis results showed that TNM staging, lymph node metastasis, and CD4+IL-17+ and CD8+ expression have a significant effect on the outcome of PC patients (P<0.05, Table 5). The nomogram results demonstrated that the survival of PC patients with TNM stage III–IV, lymph node metastasis, high CD4+IL-17+ expression, and low CD8+ expression was further reduced, as shown in Figure 3. The representative images for expression of CD4, IL-17 and CD8 in patients with 3-year survival and death outcomes were shown in Figure 4.
Table 5
| Factors | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.735 | 0.391 | 3.531 | 0.060 | 2.086 | 0.969–4.490 |
| Tumor size | 0.691 | 0.417 | 2.750 | 0.097 | 1.996 | 0.882–4.517 |
| Tumor site | 0.117 | 0.474 | 0.061 | 0.805 | 1.124 | 0.444–2.844 |
| Histological grade | 0.595 | 0.459 | 1.682 | 0.195 | 1.813 | 0.738–4.457 |
| TNM stage | 2.395 | 1.080 | 4.919 | 0.027 | 10.964 | 1.321–91.002 |
| Lymph node metastasis | 2.201 | 1.076 | 4.187 | 0.041 | 9.030 | 1.097–74.332 |
| Local infiltration | 0.774 | 0.439 | 3.103 | 0.078 | 2.168 | 0.916–5.129 |
| CD4+IL-17+ | 0.259 | 0.118 | 4.789 | 0.029 | 1.295 | 1.027–1.633 |
| CD8+T | −0.286 | 0.069 | 17.176 | <0.001 | 0.751 | 0.656–0.860 |
PC, pancreatic cancer; β, regression coefficient; SE, standard error; Wald, test statistic; OR, odds ratio; 95% CI, 95% confidence interval; TNM, tumor node metastasis.

Discussion
PC has the biological characteristics of high invasiveness and metastatic potential. Currently, there is no effective early diagnosis method. The surgical resection rate is between 10% to 20% (15,16). Most patients still have early recurrence and metastasis, and the MST is only about 18 months (17). Therefore, it is particularly important to analyze the pathogenesis of PC, and to evaluate the prognosis of patients and find therapeutic targets (18). According to relevant data, the poor prognosis and high mortality of PC are closely related to the tumor microenvironment (19,20). The microenvironment of normal tissues can effectively inhibit tumor growth and is an important barrier against tumors. Malignant solid tumor tissue can induce a state of low pH, hypoxia, and nutrient deficiency in the microenvironment by recruiting fibroblasts and regulating anti-tumor immune cells, and this is strongly linked to the tumor-suppressing effect of immune cells and the sensitivity to chemotherapeutics. Tumor immunotherapy can eliminate tumors by activating and strengthening the immune system. The immune response regulated by T cells has a significant effect on tumor immunotherapy.
Tumor-infiltrating lymphocytes are one of the major participants in the tumor microenvironment. These infiltrating cells and the secreted cytokines constitute the main components of the tumor immune microenvironment and play a role in the immune regulation of tumors (21). There are many types of immune cells in the microenvironment of PC, and CD4+ T and CD8+ T cell are main representative immune subsets of the microenvironment serving as a principal component for effective antitumor immune response. Meanwhile, the proportion of these cells can gradually become unbalanced in the process of tumor progression, making regulatory T cells (Tregs) with immunosuppressive function and tumor-associated macrophages more active and abundant by polarizing into two functionally contrasting subtypes, namely classical activated M1 macrophages and alternatively activated M2 macrophages (22). However, anti-tumor immune cells cannot play their role due to apoptosis, which is part of the immunosuppressive microenvironment of PC and becomes an important reason for treatment failure (23). After initial CD4+ T cells are stimulated by antigens, they differentiate into 3 types of effector CD4+ T cells: Th1, Th2, and Tregs.
In recent years, researchers have discovered CD4+IL-17+ T cells, a new type of effector CD4+ T cell, in encephalomyelitis and collagen-induced arthritis mouse models. Through clinical trials, it has been confirmed that CD4+IL-17+ T cells and their effector IL-17 have a significant influence on autoimmune diseases and infectious diseases (24,25). Besides, some researchers have discovered that the percentage of CD4+IL-17+ T cells in ovarian cancer tissues was higher than that in normal samples, and had a strong relationship with clinical staging (26). In addition, the proportion of CD4+IL-17+ T cells in different cancerous states of cervical cancer showed a gradually increasing trend, and was significantly correlated with clinical stage, vascular invasion, and other clinicopathological characteristics (27). CD8+ T cells have a significant impact on tumor immunity and play an important role in tumor immune surveillance. Initial CD8+ T cells can differentiate into cytotoxic CD8+ T cells under the stimulation of cancer cell antigens, which can kill antigen-specific cancer cells and inhibit tumorigenesis and development (28,29). Similar to previous reports, this study showed that the content of CD4+IL-17+ T cells in PC tissue was higher while the content of CD8+ T cells was lower than that in normal samples. This demonstrated that CD4+IL-17+ T cells may exhibit tumor-promoting effects in the microenvironment of PC, while CD8+ T cells may show tumor-inhibiting effects. In addition, Cox regression model analysis demonstrated that the TNM stage, lymph node metastasis, and CD4+IL-17+ and CD8+ T cell numbers were influencing factors for the prognosis of PC patients. These factors may become indicators for evaluating the clinical outcome of PC patients. The establishment of the nomogram model showed that the survival probability of patients with TNM stage III–IV, lymph node metastasis, high CD4+IL-17+ expression, and low CD8+ expression was further reduced.
In conclusion, CD4+IL-17+ and CD8+ T cells in PC tissue are associated with TNM staging, lymph node metastasis, and the MST. They can be used as new predictors of the prognosis of PC patients. However, this study regarding the tumor immune microenvironment lacks comprehensive data, and the study is limited by relatively small case numbers. In our subsequent trials and studies, a more convincing theoretical and experimental basis on the immune response is needed to investigate its impact on the outcome of PC.
Acknowledgments
Funding: This research received funding from the National Natural Science Foundation of China (Nos. 81873589 and 82000614).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-45/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-45/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of the Third Xiangya Hospital [No. SYXK(Xiang)2017-0002] and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu Z, Wu P, Du N, et al. Surgical choice of proximal gastric cancer in China: a retrospective study of a 30-year experience from a single center in China. Expert Rev Gastroenterol Hepatol 2019;13:1123-8. [Crossref] [PubMed]
- Moore A, Donahue T. Pancreatic Cancer. JAMA 2019;322:1426. [Crossref] [PubMed]
- DeLeo AB, Appella E. The p53 Saga: Early Steps in the Development of Tumor Immunotherapy. J Immunol 2020;204:2321-8. [Crossref] [PubMed]
- De Sanctis F, Lamolinara A, Boschi F, et al. Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. J Immunother Cancer 2022;10:e003549. [Crossref] [PubMed]
- Yang B, Zhou M, Wu Y, et al. The Impact of Immune Microenvironment on the Prognosis of Pancreatic Ductal Adenocarcinoma Based on Multi-Omics Analysis. Front Immunol 2021;12:769047. [Crossref] [PubMed]
- Opitz FV, Haeberle L, Daum A, et al. Tumor Microenvironment in Pancreatic Intraepithelial Neoplasia. Cancers (Basel) 2021;13:6188. [Crossref] [PubMed]
- Elbaz EM, Amin HAA, Kamel AS, et al. Immunomodulatory effect of diallyl sulfide on experimentally-induced benign prostate hyperplasia via the suppression of CD4+T/IL-17 and TGF-β1/ERK pathways. Inflammopharmacology 2020;28:1407-20. [Crossref] [PubMed]
- Hou Y, Guo X, Zhang C, et al. Protective effects of Jiayan Kangtai granules on autoimmune thyroiditis in a rat model by modulating Th17/Treg cell balance. J Tradit Chin Med 2018;38:380-90. [Crossref] [PubMed]
- Zhang Y, Chandra V, Riquelme Sanchez E, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med 2020;217:e20190354. [Crossref] [PubMed]
- Marques HS, de Brito BB, da Silva FAF, et al. Relationship between Th17 immune response and cancer. World J Clin Oncol 2021;12:845-67. [Crossref] [PubMed]
- Wang Z, Zhao J, Zhao H, et al. Infiltrating CD4/CD8 high T cells shows good prognostic impact in pancreatic cancer. Int J Clin Exp Pathol 2017;10:8820-8. [PubMed]
- Kumai T, Mizukoshi E, Hashiba T, et al. Effect of adoptive T-cell immunotherapy on immunological parameters and prognosis in patients with advanced pancreatic cancer. Cytotherapy 2021;23:137-45. [Crossref] [PubMed]
- MacNeil T, Vathiotis IA, Shafi S, et al. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes, Cancer-Associated Fibroblasts, and CD200 in Pancreatic Cancer. Cancers (Basel) 2021;13:5501. [Crossref] [PubMed]
- Raghavan S, Winter PS, Navia AW, et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 2021;184:6119-6137.e26. [Crossref] [PubMed]
- Noda Y, Tomita H, Ishihara T, et al. Prediction of overall survival in patients with pancreatic ductal adenocarcinoma: histogram analysis of ADC value and correlation with pathological intratumoral necrosis. BMC Med Imaging 2022;22:23. [Crossref] [PubMed]
- Keith SW, Maio V, Arafat HA, et al. Angiotensin blockade therapy and survival in pancreatic cancer: a population study. BMC Cancer 2022;22:150. [Crossref] [PubMed]
- Moningi S, Abi Jaoude J, Kouzy R, et al. Impact of Fiducial Marker Placement Before Stereotactic Body Radiation Therapy on Clinical Outcomes in Patients With Pancreatic Cancer. Adv Radiat Oncol 2020;6:100621. [Crossref] [PubMed]
- Junttila MR, Devasthali V, Cheng JH, et al. Modeling targeted inhibition of MEK and PI3 kinase in human pancreatic cancer. Mol Cancer Ther 2015;14:40-7. [Crossref] [PubMed]
- Kim H, Lin Q, Glazer PM, et al. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res 2018;20:16. [Crossref] [PubMed]
- Kennedy AL, Adams PD, Morton JP. Ras, PI3K/Akt and senescence: Paradoxes provide clues for pancreatic cancer therapy. Small GTPases 2011;2:264-7. [Crossref] [PubMed]
- Zhang J, Wang YF, Wu B, et al. Intraepithelial Attack Rather than Intratumorally Infiltration of CD8+T Lymphocytes is a Favorable Prognostic Indicator in Pancreatic Ductal Adenocarcinoma. Curr Mol Med 2017;17:689-98. [Crossref] [PubMed]
- Pan Y, Yu Y, Wang X, et al. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol 2020;11:583084. [Crossref] [PubMed]
- Chen C, Liu JM, Luo YP. MicroRNAs in tumor immunity: functional regulation in tumor-associated macrophages. J Zhejiang Univ Sci B 2020;21:12-28. [Crossref] [PubMed]
- Motrich RD, Breser ML, Molina RI, et al. Patients with chronic prostatitis/chronic pelvic pain syndrome show T helper type 1 (Th1) and Th17 self-reactive immune responses specific to prostate and seminal antigens and diminished semen quality. BJU Int 2020;126:379-87. [PubMed]
- Napier RJ, Lee EJ, Vance EE, et al. Nod2 Deficiency Augments Th17 Responses and Exacerbates Autoimmune Arthritis. J Immunol 2018;201:1889-98. [Crossref] [PubMed]
- Yan T, Li F, Chen F. Imbalance of Th1, Th17, and Treg cells in peripheral blood of patients with lymphocutaneous sporotrichosis: a comparative study. Eur J Dermatol 2020;30:345-51. [Crossref] [PubMed]
- Elkhawaga AA, Hosni A, Zaky DZ, et al. Association of Treg and TH17 Cytokines with HCV Pathogenesis and Liver Pathology. Egypt J Immunol 2019;26:55-63. [PubMed]
- Milner JJ, Toma C, Yu B, et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 2017;552:253-7. [Crossref] [PubMed]
- Jensen SS, Tingstedt JL, Larsen TK, et al. HIV-Specific CD8+ T Cell-Mediated Viral Suppression Correlates With the Expression of CD57. J Acquir Immune Defic Syndr 2016;71:8-16. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)



