The use of postoperative radiation after nipple sparing mastectomy
Introduction
Post-mastectomy radiation therapy (PMRT) has become increasingly common for women with locally advanced breast cancer. Irradiation is typically delivered to the chest wall and regional lymphatics. Current recommendations include high risk patients with a tumour size of 5 cm or larger (the cancer can be one lump, or a series, or even microscopic disease that together reach this size) and/or with at least four positive nodes in the axilla (1). Also patients with positive margin of resection (R1/R2, without possibility of achieving clear margins) or with skin involvement are suggested to receive postoperative irradiation.
Conservative surgery has also become more frequent, in order to reduce the psychological drawbacks of the mastectomy in breast cancer treatment. Conservative surgery is now well accepted not only for small tumours but also for larger tumours when oncoplastic surgery can be applied to reshape the breast despite the large defect. However, to date 25% or more of the women with breast cancer are still candidate to mastectomy because of the large size of the tumour (or the small size of the breast), or the multi-centricity of the cancer, or the local recurrence after a previous conservative treatment. To reduce the detrimental psychological effect of mastectomy, the skin-sparing mastectomy have been introduced and demonstrated to be effective and safe (2). In order to further improve the aesthetics and psychological results, the additional preservation of the nipple-areola complex (NAC) has been also described, introducing the concept of nipple-sparing mastectomy (NSM) (3). Because the indications of NSM have been progressively extended to larger or multi-centric tumours (4), this procedure has been criticised because of the increased risk of recurrence behind the areola due to the remaining glandular tissue, especially the terminal ducts, kept to preserve its blood supply, especially in case of more advanced tumours (5). To reduce this concern adjuvant radiation therapy (RT) after NSM should be administered in high risk patients who meet the criteria for current recommendations, but in other cases, such as in patients with intermediate risk or lower stage, there is no general consensus regarding indications and its role is still unclear (6).
The aim of this paper is to review the use of postoperative irradiation after NSM and try to focus on the still open questions in this setting.
Indications for adjuvant radiation therapy (RT) after mastectomy
PMRT has been known to substantially reduce the risk of loco-regional failure (LRF), and but also to increase disease specific survival and overall survival, particularly in patients with positive lymph nodes and adequate axillary surgery and even when systemic therapy is given (7). The absolute benefit gained from PMRT is believed greatest for those at high risk of LRF. There is a general consensus that PMRT should be considered when risk of LRF is greater than 20%, such as for patients with four or more positive axillary lymph nodes, primary tumour size 5 cm or more, T4 disease for skin involvement and positive margin (1). Almost all of these patients are candidate to receive external beam radiotherapy to the chest wall and to the supraclavicular/axillary region, less to the internal mammary chain. PMRT could be omitted in elderly patients with poor clinical conditions or co-morbidities that substantially reduce the life expentancy. These indications should be applied independently from the type of mastectomy, and also in presence of reconstruction.
Other factors may contribute to increase the risk of LRF, and particularly when more than one are present (8). These include young age, less than 40 years, premenopausal status, histological grade 3 tumours, invasive lobular cancer, presence of lympho-vascular invasion, less than six nodes removed at axillary dissection, positive lymph node ratio >20%, and significant nodal extracapsular invasion. Waiting for a definitive assessment of the impact of different molecular subtypes on LRF, currently still unclear, PMRT should be also considered in patients with earlier stages, but with two or more risk factors. Considerations of adverse histo-pathological factors have been included in the recent recommendations from the 2015 Saint Gallen Breast Cancer Conference (9).
It remains unclear whether patients with one to three axillary nodes positive benefit significantly from PMRT. A subset analysis of the Danish 82 b and c studies including only those patients with eight or more axillary nodes removed reported a significant and equal reductions in LRF and overall survival at 15 years with PMRT in both the one to three and greater than four involved node groups (10). Also in the Early Breast Cancer Trialists’ Collaborative Group (EDCTCG) analysis on a subgroup of more than 1,300 patients with one to three positive nodes included in the randomized trials conducted between 1964 and 1986, a decrease in LRF and breast cancer mortality was found (7). Based on these data, patients with positive axillary nodes, irrespective of the number of involved lymph nodes, considered are mandatory to be treated with PMRT in some guidelines (11).
In spite of these recommendations, the role of PMRT in this setting of patients remains controversial in practice, especially in the current era of more effective systemic therapies. A very recent study showed that the effectiveness of PMRT in terms of survival for breast cancer patients even in intermediate risk category (pT1-2 and one to three tumour positive lymph nodes) is not for all patients, but depends on the combination between the number of positive lymph nodes and the tumour size (12). Using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program between 1998 and 2008, this study respectively identified 93,793 and 36,299 women in this stages who underwent mastectomy. The association of PMRT use with overall and cause-specific survival was examined using multivariable Cox models in subgroups defined by tumour stage. In the NCDB cohort, PMRT was associated with a 14% relative risk reduction in all-cause mortality among the patients with two positive lymph nodes and tumours 2-5 cm in size or three positive nodes [hazard ratio (HR), 0.86; 95% confidence interval (CI), 0.81-0.91; P<0.0001], but PMRT had no beneficial effect for the patients with one positive node or two positive nodes and tumours 2 cm in size or smaller. Analysis of the SEER cohort confirmed this heterogeneous effect, showing PMRT to be associated with a 14% relative risk reduction in breast cancer cause-specific mortality among the patients with two positive nodes and tumours 2-5 cm in size or three positive nodes (HR, 0.86; 95% CI, 0.77-0.96; P=0.007) but not in the other subgroup. To try to resolve the question of patients with one to three positive axillary nodes a phase III randomised control trial—the Selective Use of Post-operative Radiotherapy after Mastectomy (SUPREMO) trial—is currently being conducted in Europe.
Also the role of irradiation of the internal mammary nodal region is controversial. Clinical evidences of benefit have been shown in the recent results from the European Organisation for Research and Treatment of Cancer (EORTC) 22922 and the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) MA20 randomised trials. In the EORTC trial women who had a medially or centrally located primary tumour, irrespective of axillary involvement, or node-positive axilla and receiving internal mammary irradiation improved their disease-free survival (+3%; P=0.02) and reduced breast-cancer mortality (−1.9%; P=0.02), with a marginal effect on overall survival at 10-year (P=0.06) (13). In the MA-20 study, among women with node-positive or high-risk node-negative breast cancer, the addition of regional nodal irradiation to whole breast irradiation increased the rate of disease-free survival (+5%; P=0.01), again not improving overall survival rate (14). The report of these trials can be translated in a larger number of patients who traditionally have not received regional RT (high risk LN− and 1-3 LN+) in the next future will be receiving regional RT (including internal mammary chain).
Specific risk factors for local recurrence (LR) in NSM and role of radiation
Today, no prospective and comparative studies between NSM and other types of mastectomy have been published, comparing not only the cosmetic outcome with other reconstructive techniques, but also and more important, the safety and the risk of LR, especially in case of the use of NSM in locally advanced stages. The validity of a comparison of results between various hystorical series of patients who underwent NSM is questionable because of the differences in terms of selection criteria (invasive or in-situ disease), surgical technique (one-stage surgery or delayed), and use or not of adjuvant RT.
Also the use of radiation in the setting of NSM has been sparsely reported, and often without providing details with respect to indication and technique. The presence of neoplastic cells in the retro areolar area is correlated to the distance between the tumour and the areola, therefore the conservation of the areola could be proposed only for peripheral tumours, but the margins of the tumour are sometimes difficult to evaluate preoperatively. Intraoperative frozen section examination of retro areolar tissue is considered as an important step to determine the eligibility of NSM procedure. In a series of patients treated at the European Institute of Oncology in Milan 88 cases with false-negative frozen section and ten with close margins were reported (15). Despite the frozen-section negativity, the definitive histology of retro areolar tissue revealed the presence of atypia in 11 NAC (11.2%), LCIS in 20 (20.4%), invasive carcinoma in 19 (19.4%), ductal carcinoma in situ (DCIS) in 38 (38.8%), and close margins in 10 (10.2%). The median follow-up was 64 (range, 18-113) months, and median age was 44 (range, 29-64) years. The 5-year cumulative incidence of LR and NAC recurrence was 11.2% (10/98 patients) and 2.4% (2/98 patients), respectively. The two cases of NAC recurrence consisted of Paget’s disease. Analyzing the definitive results of retro areolar tissue, the 5-year cumulative incidence of LR was 42.9% (n=4) for atypia, 8.7% (n=3) for DCIS, 10% (n=2) for LCIS, 10% (n=1) for close margins, and 0% for invasive carcinoma. Intraoperative irradiation was given on the NAC in 93 cases (94.9%). Such results could be an argument in favor of a good efficacy of radiotherapy in this group of patients, at higher risk for LR, especially in case of invasive cancer where none of 19 cases with positive margins manifested a LR.
As mentioned before, the most extensive experience on the use of radiation after NSM has been conducted in Milan, where more than 2,000 patients have been treated with intraoperative irradiation. The technique has been extensively used in breast conserving surgery (16,17). The procedure starts immediately after the subcutaneous mastectomy and before the reconstruction. An electron beam is used intra operatively with an energy level appropriately chosen, more frequently 6 MeV. A total dose of 16 Gy (prescribed at the point of maximum dose) is delivered in the region of the NAC. The biologic equivalent of a single intraoperative dose is felt to be 1.5-2.5 higher than the dose delivered with conventional fractionated external irradiation and a single dose of 16 Gy corresponds to a fractionated dose of about 45 Gy for early-responding tissue (tumour cells) and of 70-80 Gy for late-responding tissues (vessels, fat, nerves). Two shielding aluminium and lead disks are placed between the NAC and the pectoralis muscle to minimize the irradiation of the thoracic wall. The chest wall protection is guaranteed both by the absorption properties of the lead and aluminium and their thickness. The sterile collimator of the accelerator is placed in the correct position in contact with the NAC in order to guarantee the coverage of the entire target volume and simultaneously to avoid any surgical wound contamination. The area that is to be irradiated (“clinical target volume”) includes the remaining glandular tissue behind the NAC and corresponds to the NAC diameter and its periphery. The placement of a layer of gauze over the areola with a hole in the middle corresponding to the nipple is also recommended, because the thickness of the gauze further improves the homogenous distribution of the dose to the nipple and to the areola. The breast reconstruction is performed immediately after the NAC irradiation using either a prosthesis or a flap.
The results of combining NSM with intraoperative radiotherapy were reported in one thousand and one patients, treated from March 2002 to November 2007 for invasive carcinoma in 82% of the cases and in situ carcinoma in 18% (18). The median follow-up time was 20 (range, 1-69) months. The total NAC necrosis was observed in 35 cases (3.5%) and partially in 55 (5.5%). In 50 patients (5%) it was removed. The median rate of the patients for global cosmetic result on a scale ranging from 0 (worst) to 10 (excellent) was 8. Only 15% of the patients reported a partial sensitivity of the NAC. Of the 14 (1.4%) LR, ten occurred close to the tumour site, all far from the NAC corresponding to the field of radiation. No recurrences were observed in the NAC. A comparison was also performed between the 800 patients who received intraoperative irradiation and the 201 who underwent delayed one-shot radiotherapy, with the same dose by electrons, on the days following the operation, and no significant outcome difference was observed.
Using this large series of patients, the same group also identified some risk factors of recurrences in the breast and the nipple areola complex (19). The more significant risk factors of LR in the breast for the patients with invasive cancer were high grade, overexpression/amplification of HER2/neu and molecular subtype luminal B. In patients with intraepithelial neoplasia the risk factors of LR in the breast and in the NAC were age (<45 years), absence of estrogen receptors, high grade, HER2/neu overexpression and high Ki-67.
In addition to the contribution of Milan experience, there are other few reports useful to define the role of RT after NSM. Currently there is only a series showing a difference in decreasing the risk of recurrence that highlights the role of RT (20). In these patients who received subcutaneous mastectomy with or without adjuvant RT, the LR rate at a median follow-up of 13 years was 8.5% and 28.4%, respectively. This percentage of LR without RT is much higher than expected, but the selection criteria included tumours larger than 3 cm, and lymph-node metastases were found in 40.3% of patients, making these patients high risk for LR.
Also complications arising as a result of NSM treatment have been studied. In a very recent report outcomes of NSM plus immediate reconstruction from 2007 to 2013 have been evaluated (21). There were 982 NSM: 816 had no radiation, 69 had prior radiation, and 97 had PMRT. Compared to breasts with no RT, both prior RT and PMRT increased overall complications (10.2% vs. 21.7% and 17.5%, P=0.003, P=0.03, respectively) and nipple loss (0.9% vs. 4.3% and 4.1%, P=0.04, P=0.02, respectively), while PMRT increased rate of reconstruction failure (2.2% vs. 8.2%, P=0.003). On multivariate regression analysis, prior RT [odds ratio (OR), 2.53, P=0.006], PMRT (OR, 2.29, P=0.015), age >55 years (OR, 2.03, P=0.04), breast volume ≥800 cm3 (OR, 1.96, P=0.04), smoking (OR, 2.62, P=0.001), and periareolar incision (OR, 1.74, P=0.03) were independent risk factors for complications requiring surgical revision. In irradiated breasts, complication rates were 13.4% without further risk factors and 17.5%, 50%, and 66.7% when 1, 2, and ≥3 additional independent risk factors were present, respectively (P<0.001). The conclusion was that although complication rates were higher in irradiated breasts, reconstruction failure and nipple/areola necrosis was infrequent and radiation RT should not be a contraindication to NSM.
Finally, the current use of radiation after NSM has been investigated in a recent report (22). Female patients who underwent NSM or non-NSM for breast cancer from 2006 to 2010 were isolated from the SEER database. A total of 112,817 patients were included: 470 (0.4%) underwent NSM and 112,347 (99.6%) underwent non-NSM. NSM patients with 0 nodes/size ≤2 cm, 0 nodes/size 2-5 cm, and unexamined axilla/size ≤2 cm had higher odds of radiation when compared with size- and node-matched mastectomy patients. Multivariate logistic regression showed that NSM patients had higher odds of radiation (OR, 2.01, P<0.001) than mastectomy patients. Radiation was given to 18% of NSM patients who did not meet NCCN guidelines according to size or lymph node involvement, compared with 6% of mastectomy patients. This may reflect a concern with leaving ductal tissue in the NAC.
In Table 1 a series of clinical studies reporting the use of RT after NSM is listed. In the great majority of these studies the details on indication and technique of the delivery of radiation is very limited. All these studies are retrospective.
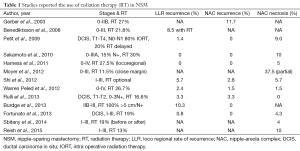
Full table
Conclusions
Recommendations for radiation delivery in breast cancer patients after mastectomy suggest that radiation is generally indicated in high-risk patients, such as those with tumour size >5 cm, positive lymph nodes in the axilla, or positive tumour margins. The definitive results of recent trials on regional irradiation can enlarge these indications to patients with intermediate risk who traditionally have not received regional radiation (high risk LN− and 1-3 LN+). The NSM is a new approach of the well known subcutaneous mastectomy which spares a small amount of glandular tissue behind the areola to protect its blood supply. Some concerns exist about the safety of these procedures, especially in case of more advanced breast tumours. The postoperative radiotherapy could complete the cancer treatment by reducing the risk of LR beneath the areola, but the use of radiation in NSM patients has been variable in the reported literature. There is a clear need for large cooperative perspective studies in this setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, Version 2015. Available online: http://www.nccn.org
- Lanitis S, Tekkis PP, Sgourakis G, et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 2010;251:632-9. [PubMed]
- Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg 2003;238:120-7. [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [PubMed]
- Petit JY, Veronesi U, Lohsiriwat V, et al. Nipple-sparing mastectomy--is it worth the risk? Nat Rev Clin Oncol 2011;8:742-7. [PubMed]
- Janssen S, Holz-Sapra E, Rades D, et al. Nipple-sparing mastectomy in breast cancer patients: The role of adjuvant radiotherapy Oncol Lett 2015;9:2435-2441. (Review). [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [PubMed]
- Rowell NP. Radiotherapy to the chest wall following mastectomy for node-negative breast cancer: a systematic review. Radiother Oncol 2009;91:23-32. [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. -Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [PubMed]
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007;82:247-53. [PubMed]
- Wenz F, Sperk E, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer IV: radiotherapy following mastectomy for invasive breast cancer. Strahlenther Onkol 2014;190:705-14. [PubMed]
- Huo D, Hou N, Jaskowiak N, et al. Use of Postmastectomy Radiotherapy and Survival Rates for Breast Cancer Patients with T1-T2 and One to Three Positive Lymph Nodes. Ann Surg Oncol 2015;22:4295-304. [PubMed]
- Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317-27. [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. [PubMed]
- Kneubil MC, Lohsiriwat V, Curigliano G, et al. Risk of locoregional recurrence in patients with false-negative frozen section or close margins of retroareolar specimen in nipple-sparing mastectomy. Ann Surg Oncol 2012;19:4117-23. [PubMed]
- Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol 2013;106:21-7. [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012;23:2053-8. [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [PubMed]
- Tang R, Coopey SB, Colwell AS, et al. Nipple-Sparing Mastectomy in Irradiated Breasts: Selecting Patients to Minimize Complications. Ann Surg Oncol 2015;22:3331-7. [PubMed]
- Agarwal S, Agarwal J. Radiation delivery in patients undergoing therapeutic nipple-sparing mastectomy. Ann Surg Oncol 2015;22:46-51. [PubMed]


