A meta-analysis examining the independent association between thyroid nodule size and malignancy
Introduction
Thyroid cancer is the most common endocrine malignancy. The incidence is rising quickly in both genders, and it was estimated to represent 3.8% of all the new cancer cases in the United States as estimated by the American Cancers Society (ACS) (1). Predicting the likelihood of malignancy is the main concern when examining any thyroid nodule. The initial evaluation for all thyroid nodules includes both high-resolution ultrasound and fine-needle aspiration (FNA) when indicated.
A combination of clinical factors and ultrasound features helps determine whether a nodule is likely to be cancerous or not. These usually include, but are not limited to, nodule hypoechogenicity, presence of calcifications, ill-defined or infiltrative margins, or increased intra-nodular vascularity (2). Nevertheless, the accuracy of these features in predicting the malignant potential of a nodule is not recognized.
Of these different variables, nodule size is easily measured. However, data examining the relationship between thyroid nodule size and malignancy risk remains inconsistent. Multiple studies have been published on the subject (3-6), but many shortcomings have been identified, including small study population (6), study populations from a single institution (7), retrospective studies (3), inconsistent results across different studies, and nodules that were rendered benign based on FNA results only. We hypothesized that a cutoff of 3 cm might be more precise in determining malignancy risk. Therefore, we conducted a meta-analysis evaluating the available evidence in the literature regarding nodule size and its relationship to malignant behavior of thyroid nodules.
Materials and methods
Search strategy
A preliminary search was conducted for articles related to nodule size and its associated risk of malignancy to evaluate the volume of the relevant bibliography and choose keywords for the main search. Two reviewers (Hammad M. Masoodi, Yasin Ibrahim) conducted an independent systematic review of articles published in electronic databases (PubMed, Embase, Medline, and Web of Science) from January 1, 1996 through June 1, 2013. Articles were identified using the following keywords: (I) thyroid nodule; (II) thyroid carcinoma; (III) thyroid malignancy; (IV) follicular neoplasm; and (V) thyroid FNA biopsy. These terms were identified in the title, abstract, or medical subject heading (MeSH). The studies were further restricted to articles written in English, and those involving adult human populations.
Data were abstracted using a standardized form. For each of the included studies, two reviewers extracted the following data: the country where the study was conducted, primary author of the study, number of patients and patient demographics, nodule size and characteristics, FNA results, and final histopathology results. Patients were divided into three major categories. Our reference category included those with nodule sizes <3 cm. The other categories were nodules sized 3–5.9 cm and those with nodules ≥6 cm. In our spreadsheet used for data collection, we recorded the incidence of malignancy for each category, P values, and study conclusion. The primary outcome was histologically proven malignancy per nodule size category.
Study selection
Articles were included in our study if they met the following criteria: (I) prospective clinical studies, retrospective or cohort analyses discussing the association between nodule size and malignancy; (II) studies where the final histopathology result for the nodules was included; (III) articles including nodules labeled indeterminate by FNA (includes follicular neoplasm and Hürthle cell neoplasm) were also included in our study analysis.
Articles excluded from our study were: (I) abstracts, posters and literature reviews; (II) articles that are not examining the association between nodule size and malignancy development; and (III) studies using non-categorical data. After obtaining a full list of studies, the same reviewers independently assessed each study for eligibility for inclusion in the meta-analysis, and data was extracted according to the study criteria into a spread sheet. Differences between reviewers were resolved by discussion, and agreement on the final dataset was reached by consensus.
Statistical analysis
We analyzed the correlation between nodule size and incidence of cancer in each of our three groups. Nodule sizes were divided and categorized into three major groups: <3, 3–5.9 and ≥6 cm nodules. Adjustments were made for age and gender, and the effect size of clinicopathologic parameters, defined as the quantitative measure of the association strength between two variables, was calculated by the means of odds ratio (OR) for malignancy in each group. A DerSimonian and Laird random effects model was used to estimate and pool data for heterogeneity testing. The presence of heterogeneity was assessed by the Q test, and the extent of heterogeneity was quantified by the Chi-squared index. P value was estimated using Fisher’s exact test and a P value less than 0.05 was considered significant. A funnel plot was constructed to assess the publication bias, where each group was compared to the reference group. The Begg’s rank correlation test was used to examine the funnel plot asymmetry, and the Egger’s weighted linear regression test was used to examine the association between the mean effect and its variance. Single studies were removed one at a time and data analysis was run to validate the heterogeneity of the data. Sensitivity analysis was performed to test the robustness of the results. The two studies originating from outside the United States were excluded, and the consistency of the results was examined. Forest plots were constructed to compare different OR between different studies and get the overall 95% confidence interval (CI). Age- and gender-adjusted OR was done as previous studies have shown that the incidence of malignancy increases in young, male patients (8). An adjusted OR for these variables was used to ensure the elimination of any bias. All analyses were conducted using SATA analytical software.
Results
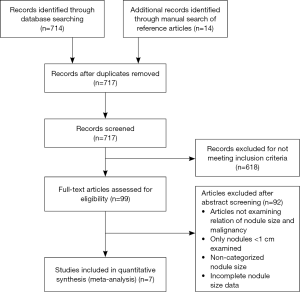
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) was used to reach a total of seven articles that met our inclusion criteria (Figure 1). All articles underwent a full-text review and were included in the final meta-analysis (3,9-14).
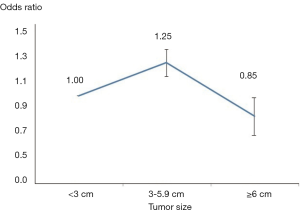
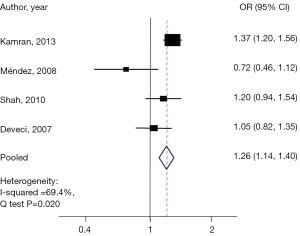
Malignancy was identified in 2,206 nodules (20.4%). Four articles evaluated the risk of malignancy in nodules 3-5.9 cm as compared to the control group, while four articles examined the risk in those with nodules ≥6 cm. After adjusting for age and sex among groups, an age- and gender-adjusted OR was plotted (Figure 2). When compared to the reference group, those with nodule size 3–5.9 cm were found to have a 25% higher malignancy risk (pooled OR, 1.26; 95% CI: 1.13–1.39). Q-testing for heterogeneity was statistically significant (P=0.02) with a Chi-squared of 69.4% (Figure 3), demonstrating a significant association between nodules 3–5.9 cm in diameter and thyroid cancer.
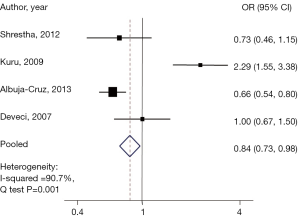
Interestingly when comparing those in the group with a nodule size ≥6 cm to the reference group, a 15% lower risk of malignancy was found (pooled OR, 0.84; 95% CI: 0.73–0.98), with a statistically significant heterogeneity Q test, (P<0.001), and a Chi-square =90.7% (Figure 4).
All of the included studies were conducted in the USA except for two, one conducted in Turkey (13), and the other in Canada (14). Following the sensitivity analysis, the results were still significant, where those with nodules between 3-5.9 cm were significantly associated with malignancy (OR, 1.28; 95% CI: 1.14–1.43). Similarly, those with nodule size ≥6 cm were still associated with lower risk of malignancy (OR, 0.71; 95% CI: 0.60–0.83) (Table 1).

Full table
Discussion
In this meta-analysis, we scrutinized published articles examining the effect of thyroid nodule size as the sole predictor of malignancy risk. We included 10,817 thyroid nodules, making this one of the largest analyses of thyroid nodule size and cancer risk to date. The results of this meta-analysis suggest that nodule size may predict cancer risk. A recently published article by Shin et al. group suggests that surgery represents a reasonable option for nodules 3 to 4 cm (15). In our study, patients in the nodule group of size 3–5.9 cm have a 26% higher malignancy risk compared to those <3 cm in size. On the other hand, the risk of malignancy was found to drop by 16% when nodule size is 6 cm or more. These data elucidate previously conflicting reports as to whether nodule size affects thyroid cancer risk, and could alter clinical decision making (2,16,17).
Two major concerns for many surgeons are nodules shown to be follicular neoplasms and/or Hürthle cell on FNA cytology. Follicular neoplasms represent a challenge as FNA cytology cannot differentiate between follicular carcinoma and follicular adenoma, requiring a thorough pathological examination. A nodule suspicious for follicular neoplasm may carry a risk of malignancy of 20–30% (18). Hürthle cell neoplasms have often been considered a more aggressive variant of follicular carcinoma due to their early metastasis and aggressive lymphovascular invasion (19). The 2009 American Thyroid Association guidelines recommend thyroid lobectomy for suspicious solid lesions of follicular neoplasms or Hürthle cell carcinomas without accompanying risk factors, and total thyroidectomy for tumor size >4 cm or displaying signs of atypia. Large nodules size are associated with false negative results (16), and are typically difficult to sample entirely. The 2009 guidelines did not differentiate between nodules ≥6 cm and those measuring 4–6 cm. Our study confirms the previous guidelines showing an increased risk of cancer in patients with nodules 3–5 cm, while pointing out the lower risk of malignancy seen in patients with nodules ≥6 cm.
This study is not without shortcomings. First, one of the included studies contributed with over 50% of the examined nodules (3). Although the risk of bias in each study was examined and our results were adjusted according to the age, sex, clinicopathologic diameters, yet, the effect of this study on the overall results remains to be considered. Additionally, another study excluded patients with nodules <1 cm from the <3 cm group (3). The effect of this exclusion might affect the overall results reported by this group as subcentimeter malignancies exist and can affect the total number of malignancies reported in the <3 cm category.
Based on our results, we propose an additional cutoff point of 6 cm. This is where the incidence of carcinoma decreases with larger size thyroid nodules. Therefore, if the patients in this group choose to undergo surgery for compressive symptoms or other reasons, a less invasive surgical procedure such as thyroid lobectomy may be offered, avoiding the certain life-long hormonal replacement needed after total thyroidectomy.
Conclusions
The size of thyroid nodules can be used as predictor for malignancy risk. Those with a nodule size between 3–5.9 cm have an increased risk of 26% when compared to those <3 cm in largest diameter. However, nodules ≥6 cm carry a lower risk for malignancy (16%, as compared to the reference group). Thoughtful consideration of the appropriate surgical intervention should be given to patients with nodules 3–5 cm in size due to their higher malignancy risk, especially if other clinical and/or ultrasonographic features suggestive of malignancy are present. While further validations are needed, our data provides important information to counsel patients and guide personalized clinical decisions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Howlader NN, Krapcho M, Garshell J, et al, eds. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute. Bethesda, MD, 2013. Available online: http://seer.cancer.gov/csr/1975_2010/
- Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002;87:1941-6. [Crossref] [PubMed]
- Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab 2013;98:564-70. [Crossref] [PubMed]
- Yang GC, Goldberg JD, Ye PX. Risk of malignancy in follicular neoplasms without nuclear atypia: statistical analysis of 397 thyroidectomies. Endocr Pract 2003;9:510-6. [Crossref] [PubMed]
- Williams MD, Suliburk JW, Staerkel GA, et al. Clinical significance of distinguishing between follicular lesion and follicular neoplasm in thyroid fine-needle aspiration biopsy. Ann Surg Oncol 2009;16:3146-53. [Crossref] [PubMed]
- Turanli S, Pirhan Y, Ozcelik CK, et al. Predictors of malignancy in patients with a thyroid nodule that contains Hürthle cells. Otolaryngol Head Neck Surg 2011;144:514-7. [Crossref] [PubMed]
- Baloch ZW, Fleisher S. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol 2002;26:41-4. [Crossref] [PubMed]
- Smith JJ, Chen X, Schneider DF, et al. Cancer after thyroidectomy: a multi-institutional experience with 1,523 patients. J Am Coll Surg 2013;216:571-7; discussion 577-9. [Crossref] [PubMed]
- Deveci MS, Deveci G. Concordance between thyroid nodule sizes measured by ultrasound and gross pathology examination: effect on patient management. Diagn Cytopathol 2007;35:579-83. [Crossref] [PubMed]
- Albuja-Cruz MB, Goldfarb M, Gondek SS, et al. Reliability of fine-needle aspiration for thyroid nodules greater than or equal to 4 cm. J Surg Res 2013;181:6-10. [Crossref] [PubMed]
- Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine-needle aspiration: a 10-year study from a single institution. Thyroid 2012;22:1251-6. [Crossref] [PubMed]
- Méndez W, Rodgers SE, Lew JI, et al. Role of surgeon-performed ultrasound in predicting malignancy in patients with indeterminate thyroid nodules. Ann Surg Oncol 2008;15:2487-92. [Crossref] [PubMed]
- Kuru B, Gulcelik NE, Gulcelik MA, et al. he false-negative rate of fine-needle aspiration cytology for diagnosing thyroid carcinoma in thyroid nodules. Langenbecks Arch Surg 2010;395:127-32. [Crossref] [PubMed]
- Shah MD, Conrad A, Ahmed A, et al. Decision making for the extent of thyroidectomy in the patient with atypical cytologic results. Arch Otolaryngol Head Neck Surg 2010;136:1177-80. [Crossref] [PubMed]
- Shin JJ, Caragacianu D, Randolph GW. Impact of thyroid nodule size on prevalence and post-test probability of malignancy: a systematic review. Laryngoscope 2015;125:263-72. [Crossref] [PubMed]
- Leenhardt L, Hejblum G, Franc B, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab 1999;84:24-8. [Crossref] [PubMed]
- Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist 2008;13:105-12. [Crossref] [PubMed]
- Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36:425-37.
- Eng CY, Quraishi MS, Bradley PJ. Management of Thyroid nodules in adult patients. Head Neck Oncol 2010;2:11. [Crossref] [PubMed]