
Intraglandular dissemination is a risk factor for lymph node metastasis in papillary thyroid carcinoma: a propensity score matching analysis
Introduction
Thyroid cancer (THCA) is the most common endocrine malignancy (1) and owing to the increased use of diagnostic imaging and surveillance, is projected to become the fourth leading type of cancer across the globe (2,3). Papillary thyroid carcinoma (PTC) is the most frequent subtype, accounting for 85–90% of all thyroid tumors (4,5). Although PTC is considered an indolent tumor and has the best overall prognosis, central lymph node (LN) metastases occur in 30–80% of patients (6), and lateral LN metastases are found in 35.2–44.5% of patients (7). Moreover, up to approximately 14.9% of differentiated thyroid cancer (DTC) patients develop disease persistence or recurrence after initial treatments, leading to an increased rate of reoperation. Therefore, identifying prognostic risk factors for PTC would contribute to the development of more effective management strategies for patients and decreasing the incidence of reoperation. Cervical LN metastases are not only an important indicator for evaluating PTC prognosis, scope, and method of surgery, but they also serve as an independent risk factor for patients with a high recurrence rate and low survival rate (8,9). Studies have shown that tumor size, tumor extension, tumor location, and microcalcifications are significantly correlated with a high rate of LN metastasis (10,11). However, a proportion of patients without these risk factors are sometimes found to have cervical LN metastases at the time of surgery and in pathology specimens.
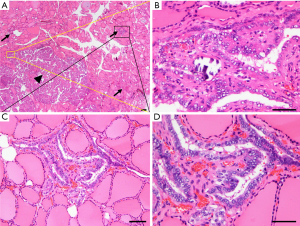
Intraglandular dissemination is considered a means of metastasis in THCA, in which cancer cells spread through the lymphatic ducts within the thyroid gland. The microscopic pathological features of intraglandular dissemination are as follows: (I) the disseminated lesions show the same histological characteristics as the main cancer nodules, but the volume is smaller; (II) the disseminated lesions present as a large number of small lesions radiating around the main cancer focus; (III) the farther the disseminated lesions deviate from the main carcinoma, the smaller the volume and the lower the density; (IV) the thyroid tissue around the disseminated lesions does not show any pathological abnormalities (such as sclerotic fibrous stroma or a fibrous capsule); and (V) depending on the progression of the disease, the disseminated lesions may occur at any location within the bilateral thyroid gland. A representative pathological image is shown in Figure 1. Based on these facts, it is possible to distinguish between intraglandular dissemination of PTC and multifocal PTC (MPTC). Additionally, patients with pathologically confirmed intraglandular dissemination seem to be more likely to develop cervical LN metastases. However, statistical evidence of the relationship between intraglandular dissemination and various clinicopathological parameters has not been widely reported in the literature. Clarifying this relationship would facilitate the personalized assessment and better management of PTC patients.
In retrospective observational studies, participants in treatment and control groups may differ due to confounders, while biases in outcomes can reflect differences in baseline conditions rather than an actual treatment effect (12). To address this, we used propensity score matching (PSM), a method of ensuring an even distribution of confounders and biases between treatment and control groups, thereby increasing group comparability (13). Our aim was to retrospectively investigate the correlation between intraglandular dissemination of PTC and various clinicopathological parameters by means of PSM.
We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at https://dx.doi.org/10.21037/gs-21-470).
Methods
Data source and patient selection
A retrospective study was performed by recruiting patients from the Thyroid and Breast Disease Center database of Wuhan Union Hospital. The inclusion criteria included patients who were admitted between 1 June 2020 and 1 May 2021 and were pathologically diagnosed with PTC. Those who met any of the following conditions were excluded: (I) missing baseline data, and (II) patients for whom the surgical procedure was not the primary surgery. The data collected in this study included patient ID, gender, age, and postoperative pathological report [tumor subtype, tumor diameter, number of tumor foci, cervical LN dissection results, presence of intraglandular dissemination and extrathyroidal extension, and presence of accompanying Hashimoto’s thyroiditis (HT)]. The surgical procedures included total thyroidectomy with bilateral central LN dissection (CLND), unilateral thyroid lobectomy with CLND, or total thyroidectomy with bilateral CLND and lateral cervical LN dissection of the affected side.
Variable recoding and group assignment
The variables selected in this study included age, gender, histological subtypes, tumor size, multifocality, extrathyroidal extension, presence of HT, LN metastasis rate, number of metastatic LNs, and intraglandular dissemination. Linear variables conforming to the normal distribution were expressed as mean (SD), and the categorical variables were expressed as number (frequency). Participants were divided into 2 groups based on whether PTC was accompanied by intraglandular dissemination.
Analysis procedures
First, the differences in clinicopathological characteristics between the 2 groups were statistically compared. Next, PSM was applied to balance potential baseline confounders and biases between the 2 groups, and the clinicopathological differences between the groups were further verified (14). Finally, logistic regression analysis was performed to quantify the association between intraglandular dissemination and cervical LN metastasis.
Statistical analysis
Fisher’s exact test and a chi-square test were used to analyze categorical variables, while a t-test was applied to compare continuous variables. All statistical analyses were performed using RStudio version 4.0.3 (http://www.r-project.org). The “matchit” package in RStudio was used to match the propensity score between the groups (15). The matching method was set to the nearest neighbor algorithm, ratio was set at 1:1 or 2:1, and caliper value was set at 0.05 or 0.02 depending on whether PTC occurred with or without intraglandular dissemination, respectively (16). The “cobalt” package in RStudio was used to estimate kernel density and analyze the standardized mean difference (SMD) in order to assess the covariate balance in the matched groups (17). Variables with standardized differences of <10% between the 2 groups were considered well-balanced after PSM (18). All P values were 2 sided, and a P value of <0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the Union Hospital, Tongji Medical College of Huazhong University of Science and Technology (No. 0304-01). Due to the retrospective nature of the study, participant informed consent was waived.
Results
Demographic and clinical features
Initially, 1,071 potentially relevant cases were identified, and a total of 1,043 PTC patients were collected and analyzed according to the inclusion criteria. The specific results and screening process are shown in Figure 2. The baseline characteristics of the selected patients are presented in Table 1. Among the cases included, 138 (13.2%) patients with PTC had intraglandular dissemination and 905 (86.8%) did not, indicating an incidence of intraglandular dissemination of about 13.2% in this cohort. The mean age of participants in the intraglandular dissemination group was 35.93 (SD =10.98) years and the average tumor size was about 12.22 mm (SD =8.84), while the corresponding values of the non-intraglandular dissemination group were 42.62 (SD =11.20) years and 8.33 mm (SD =6.68), respectively. The male-to-female ratio in all cases was about 1:3, and the incidence of multifocality, extrathyroidal extension, and HT was 42.1%, 59.2%, and 38%, respectively. A total of 121 (87.7%) participants in the intraglandular dissemination group had LN metastases, and the average number of metastatic LNs was 10.54 (SD =8.71). The corresponding values in the non-intraglandular dissemination group were 478 (52.8%) and 2.30 (SD =3.98), respectively. These results suggested that intraglandular dissemination might be a promoter of LN metastases.
Table 1
| Covariate | Total | PTC with intraglandular dissemination | P value | |
|---|---|---|---|---|
| No | Yes | |||
| Sample, n (%) | 1,043 (100.0) | 905 (86.8) | 138 (13.2) | – |
| Age, years [mean (SD)] | 41.73 (11.39) | 42.62 (11.20) | 35.93 (10.98) | <0.001 |
| Sex, n (%) | ||||
| Female | 781 (74.9) | 698 (77.1) | 83 (60.1) | <0.001 |
| Male | 262 (25.1) | 207 (22.9) | 55 (39.9) | |
| Histological subtypes, n (%) | <0.001 | |||
| Classical | 840 (80.5) | 711 (78.6) | 129 (93.5) | |
| Follicular | 82 (7.89) | 80 (8.8) | 2 (1.4) | |
| Others | 121 (11.6) | 114 (12.6) | 7 (5.1) | |
| Tumor size, mm [mean (SD)] | 8.84 (7.11) | 8.33 (6.68) | 12.22 (8.84) | <0.001 |
| Multifocality, n (%) | <0.001 | |||
| Yes | 439 (42.1) | 358 (39.6) | 81 (58.7) | |
| No | 604 (57.9) | 547 (60.4) | 57 (41.3) | |
| Extrathyroidal extension, n (%) | <0.001 | |||
| Yes | 617 (59.2) | 506 (55.9) | 111 (80.4) | |
| No | 426 (40.8) | 399 (44.1) | 27 (19.6) | |
| Hashimoto’s thyroiditis, n (%) | <0.001 | |||
| Yes | 396 (38.0) | 368 (40.7) | 28 (20.3) | |
| No | 647 (62.0) | 537 (59.3) | 110 (79.7) | |
| LN metastasis, n (%) | <0.001 | |||
| Yes | 599 (57.4) | 478 (52.8) | 121 (87.7) | |
| No | 444 (42.6) | 427 (47.2) | 17 (12.3) | |
| No. of metastatic LN [mean (SD)] | 3.39 (5.617) | 2.30 (3.98) | 10.54 (8.71) | <0.001 |
PTC, papillary thyroid carcinoma; SD, standard deviation; LN, lymph node; No, number.
PSM adjustment of patient characteristics
A preliminary analysis of the original data showed that there were statistically significant differences between PTC patients with and without intraglandular dissemination regarding age, gender, histological subtypes, tumor size, multifocality, extrathyroidal extension, presence of HT, and LN metastases (all P values <0.001; Table 1). Given the observed heterogeneity in baseline characteristics and the inherent potential biases of any retrospective study, PSM was applied to reduce the impact of confounders between the cohort data (19). Patients with intraglandular dissemination were 1:2 propensity matched with a caliper value of 0.05 to yield 117 matched pairs among 321 patients. The clinicopathological comparison between the groups is presented in Table 2. After matching, the cohorts did not significantly differ in terms of age (P=0.865), gender (P=0.799), histological subtype (P=0.965), tumor size (P=0.893), multifocality (P=0.499), extrathyroidal extension (P=0.819), or presence of HT (P=0.375). However, the difference in LN metastases between PTC patients with and without intraglandular dissemination remained significant, indicating that intraglandular dissemination was highly correlated with a higher rate of LN metastases (88% vs. 67.2%, P<0.001) and a greater number of metastatic LNs (9.62, SD =7.92 vs. 3.33, SD =4.23; P<0.001). We adjusted either the matching ratio to 1:1 or the caliper value to 0.02, and the results remained consistent with those reported above. The results of the cohort comparison using different parameter settings of PSM are presented in Table 3. In addition, logistic regression analysis suggested that intraglandular dissemination was associated with an increased risk of LN metastasis in both the unmatched patients (OR, 3.19; 95% CI: 1.74 to 5.86; P<0.001) and the matched subset (OR, 4.00; 95% CI: 1.98 to 8.05; P<0.001) (Table 4).
Table 2
| Covariate | Total | PTC with intraglandular dissemination | P value | |
|---|---|---|---|---|
| No | Yes | |||
| Sample, n (%) | 321 (100.0) | 204 (63.6) | 117 (36.4) | |
| Age, years [mean (SD)] | 36.68 (10.05) | 36.75 (9.88) | 36.55 (10.38) | 0.865 |
| Sex, n (%) | 0.799 | |||
| Female | 210 (65.4) | 135 (66.2) | 75 (64.1) | |
| Male | 111 (34.6) | 69 (33.8) | 42 (35.9) | |
| Follicular, n (%) | 4 (1.2) | 2 (1.0) | 2 (1.7) | 0.965 |
| Tumor size, mm [mean (SD)] | 10.99 (8.19) | 10.91 (8.96) | 11.04 (6.69) | 0.893 |
| Multifocality, n (%) | 0.499 | |||
| Yes | 158 (49.2) | 97 (47.5) | 61 (52.1) | |
| No | 163 (50.8) | 107 (52.5) | 56 (47.9) | |
| Extrathyroidal extension, n (%) | 0.819 | |||
| Yes | 246 (76.6) | 155 (76.0) | 91 (77.8) | |
| No | 75 (23.4) | 49 (24.0) | 26 (22.2) | |
| Hashimoto’s thyroiditis, n (%) | 0.375 | |||
| Yes | 79 (24.6) | 54 (26.5) | 25 (21.4) | |
| No | 242 (75.4) | 150 (73.5) | 92 (78.6) | |
| LN metastasis, n (%) | <0.001 | |||
| Yes | 240 (74.8) | 137 (67.2) | 103 (88.0) | |
| No | 81 (25.2) | 67 (32.8) | 14 (12.0) | |
| No. of metastatic LN, [mean (SD)] | 5.62 (6.10) | 3.33 (4.23) | 9.62 (7.92) | <0.001 |
PTC, papillary thyroid carcinoma; SD, standard deviation; LN, lymph node.
Table 3
| Covariate | Pre-PSM, P value | Post-PSM | Post-PSM | ||||
|---|---|---|---|---|---|---|---|
| P value (caliper value =0.05) | P value (caliper value =0.02) | ||||||
| Ratio (1:1) | Ratio (2:1) | Ratio (1:1) | Ratio (2:1) | ||||
| Sample | 905:138 | 117:117 | 204:117 | 105:105 | 182:105 | ||
| Age | <0.001 | 0.891 | 0.865 | 1 | 0.977 | ||
| Sex | <0.001 | 0.666 | 0.799 | 0.426 | 0.886 | ||
| Histological subtypes | <0.001 | 1 | 0.965 | 1 | 0.969 | ||
| Tumor size | <0.001 | 0.497 | 0.893 | 0.428 | 0.708 | ||
| Multifocality | <0.001 | 0.793 | 0.499 | 1 | 0.407 | ||
| Extrathyroidal extension | <0.001 | 1 | 0.819 | 0.871 | 0.953 | ||
| Hashimoto’s thyroiditis | <0.001 | 0.178 | 0.375 | 0.352 | 0.403 | ||
| LN metastasis | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| No. of metastatic LN | <0.001 | 0.003 | <0.001 | 0.002 | <0.001 | ||
PSM, propensity score matching; LN, lymph node.
Table 4
| Covariate | Pre-PSM | Post-PSM | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 0.36 (0.28–0.47) | <0.001 | 0.37 (0.23–0.58) | <0.001 | |
| Sex | <0.001 | <0.001 | |||
| Male | 2.77 (1.88–4.07) | 3.57 (1.79–7.14) | |||
| Female | Ref. | Ref. | |||
| Tumor size | 2.13 (1.69–2.70) | <0.001 | 3.11 (1.86–5.19) | <0.001 | |
| Multifocality | 0.297 | 0.338 | |||
| Yes | 1.19 (0.86–1.64) | 1.36 (0.73–2.54) | |||
| No | Ref. | Ref. | |||
| Extrathyroidal extension | 0.001 | 0.643 | |||
| Yes | Ref. | Ref. | |||
| No | 0.59 (0.43–0.82) | 0.85 (0.42–1.71) | |||
| Hashimoto’s thyroiditis | 0.186 | 0.593 | |||
| Yes | 0.81 (0.59–1.11) | 0.83 (0.42–1.63) | |||
| No | Ref. | Ref. | |||
| Intraglandular dissemination | <0.001 | <0.001 | |||
| Yes | 3.19 (1.74–5.86) | 4.00 (1.98–8.05) | |||
| No | Ref. | Ref. | |||
PSM, propensity score matching; OR, Odds Ratio; 95% CI, 95% confidence interval.
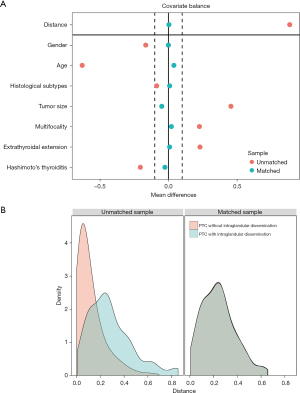
Assessment of the covariate balance in the matched groups
Assessment of the covariate balance in the matched groups was an important step in determining the quality of the resulting matched samples (20). The balance in this study was defined as similarity in the empirical distributions of the full set of covariates between the matched intraglandular dissemination and control groups. Consistent with Rubin (21), the mean standardized difference analysis showed that all the covariates were less than 10%, which indicated a well-balanced result (14) (Figure 3A). Moreover, kernel density estimation (Figure 3B) indicated that the cohort presented a low degree of overlap before matching, but an adequate overlap after matching, with a good control match for each treated individual. Therefore, the above results jointly confirmed that the confounders and the biases between the groups were well balanced and the comparison was reliable.
Discussion
In addition to being the most common pathological type of THCA, PTC is one of the fastest growing malignancies (22). Although PTC usually presents a better prognosis than other tumors, LN metastases are common (23). More importantly, studies have confirmed that LN metastases in PTC are associated with compromised survival (24). Therefore, the assessment and screening of metastases is an important part of the management of patients with PTC. However, in one study, nearly 27.6% of patients with PTC had microscopic LN disease that was not clinically detectable by preoperative imaging and intraoperative inspection (25). According to the American Thyroid Association (ATA) guidelines, performing a prophylactic CLND for PTC patients with cN0 (clinically uninvolved central LN metastases) is still considered controversial (26). Exploring the risk factors related to cervical LN metastases will improve the risk stratification of patients with PTC, optimize surgical strategy and the selection of radioactive iodine, and help reduce recurrence and mortality.
In the present study, we retrospectively collected 1,043 cases of PTC and used PSM to clarify the significant correlation between intraglandular dissemination of PTC and a higher rate and number of LN metastases. The reliability of the results was verified by the assessment of the covariate balance in the matched groups. This was the first study of PTC to use PSM to correct for confounding variables.
Definition and diagnosis of intraglandular dissemination of PTC
Intraglandular dissemination of PTC is considered a metastatic result of the spread of cancer cells through lymphatic vessels within the gland (27). So far, there is no authoritative report on the definition or diagnostic criteria of intraglandular dissemination of PTC. In this study, we preliminarily defined intraglandular dissemination as microscopic cancer lesions that are radially distributed around and present a similar histological appearance to the main cancer nodule. As the distribution distance increases, the number and volume of the satellite cancer lesions decreases gradually. As shown in Figure 1, multiple satellite cancer lesions with a similar histological appearance can be seen radially distributed around the main cancer focus. These disseminated lesions are usually less than 4 mm in size and not accompanied by sclerotic fibrous stroma or a fibrous capsule (28). According to Iida et al., no histologic continuity can be seen between the disseminated lesions and the main cancer nodule (27).
The previously reported incidence of intraglandular dissemination has varied greatly, ranging from 18% to 87.5% (29,30). Katoh et al. sectioned the whole thyroid resected by thyroidectomy at 2–3 mm intervals and found that intraglandular dissemination occurred in 80 out of 105 cases (28). In a study of 328 cases of THCA, Black et al. found that about 20% were multicentric (30). In the present study, the incidence of intraglandular dissemination confirmed by postoperative pathology was approximately 13.2%. Reported data fluctuations may be due to the inconsistent diagnostic criteria or different pathological sampling ranges. The lower incidence in the present study may also be due to the earlier diagnosis of PTC.
Intraglandular dissemination of PTC should be distinguished from that of MPTC. Jin et al. noted that the surrounding lesions of intraglandular dissemination of PTC are smaller than those of MPTC and can only be observed under a microscope (31). They also noted that multiple means of imaging, such as ultrasound combined with computed tomography (CT) image, contributed to preoperative diagnosis, and that intraoperative frozen pathology section was a helpful guide during surgery. Katoh et al. observed that the disseminated lesions in PTC appear to be smaller and histologically nonsclerotic and are generally located in the interfollicular interstitium (28). Iida et al. demonstrated that the small foci are histologically identical to the primary cancer nodule, suggesting that the smaller tumors are intraglandular metastases of the larger tumor (27). However, based on the analysis of the patterns of X-chromosome inactivation, Shattuck et al. clarified that individual tumor foci in patients with MPTC often arise as independent tumors (32). With the help of whole-exome sequencing and targeted region sequencing, some researchers have suggested that the multiple foci may arise from either intraglandular metastases or multiple independent origins, or both (33). Based on comprehensive molecular profiling, Bansal et al. demonstrated that at least 30% and likely >60% of MPTCs are of independent clonal origin and develop through distinct mutational mechanisms (34). Whether these multifocal neoplasms are caused by multiple primary neoplasms arising from independent clones or by intraglandular metastases arising from a single malignant clone is still controversial. There is thus a necessity and an urgency for uniform pathological testing and a diagnostic standard for intraglandular metastases of PTC.
Intraglandular dissemination of PTC and LN metastasis
The PSM analysis (matching ratio, 2:1; caliper value, 0.05) revealed that the LN metastasis rate of patients with intraglandular dissemination (88%) was significantly higher than that of patients without intraglandular dissemination (67.2%; P<0.001; Table 2). The number of metastatic LNs in patients with and without intraglandular dissemination also varied greatly, at 9.62 (SD =7.92) and 3.33 (SD =4.23), respectively. These significant differences remained stable under different PSM parameters (all P values <0.001; Table 3). In addition, participants with intraglandular dissemination were associated with an increased risk of LN metastasis in both the unmatched group (OR, 3.19; 95% CI: 1.74 to 5.86) and the matched subset (OR, 4.00; 95% CI: 1.98 to 8.05; Table 4). This result is consistent with previous studies (27-29) that suggest that intraglandular dissemination is a risk factor for LN metastases in PTC. According to Shattuck et al., this phenomenon may be attributed to the thyroid’s unique lymphatic drainage system, which wraps the thyroid in a capsule containing a rich intralobular lymphatic network (32). The lymph vessels running between the thyroid follicles anastomose inside the gland and penetrate into the capsule to communicate with the vessels outside the gland (29). Cancer cells may be more likely to metastasize to residual parts of the gland and LNs around the thyroid through the intralobular lymphatic network. Studies have suggested that disseminated lesions left in the remaining thyroid tissue may be the major cause of THCA recurrence following operation (27). Therefore, we advocate more aggressive management of these patients. The surgical scope should be total thyroidectomy with CLND, even when the lesions are limited to 1 thyroid lobe.
Intraglandular dissemination of PTC and other clinicopathological parameters
In our study, intraglandular dissemination was not related to demographic parameters such as age (P=0.865) or gender (P=0.799). Tumor size, histological subtypes, extrathyroidal extension, and HT have been reported to be associated with intraglandular dissemination, but this correlation was not found in this study (P values were 0.893, 0.965, 0.819, and 0.375, respectively). Gerfo et al. concluded that small tumors (<2 cm) have a higher incidence of additional foci than larger ones (>4 cm) (35). Iida et al. observed that intraglandular dissemination of THCA is more common in follicular adenocarcinoma than in other pathologic types and that the frequency of intraglandular dissemination increases as the degree of histologic extension progresses (27). Jin et al. demonstrated that HT causes diffuse destruction of thyroid cells and increases levels of thyroid stimulating hormone (TSH), which may stimulate the proliferation of papillary cancer cells (31). Thus, HT and hereditary THCA are both risk factors for MPTC.
In this study, we found that intraglandular dissemination of PTC was associated with a higher rate of LN metastases and a greater number of metastatic LNs. Although intraglandular dissemination still lacks an authoritative pathological definition and a reliable preoperative diagnosis, it is clear that PTC patients with intraglandular dissemination may require more thorough LN dissection and closer follow-up. Therapy to suppress TSH and the use of radioiodine (sodium l-131) for these patients need to be supported by evidence-based medicine. Due to the lack of sufficient follow-up data in the present study, such as overall survival (OS) and disease-free survival (DFS), further analysis of these patients could not be performed. Relevant literature on this subject is also scarce. More in-depth studies with larger cohorts would address these issues.
Conclusions
In summary, our study found that intraglandular dissemination is a risk factor for LN metastasis in PTC, which suggests a need for more thorough LN dissection and closer follow-up in these patients. This study may provide reliable reference data relating to the risk stratification of patients with PTC.
Acknowledgments
We would like to thank the AME Editing Service (Editors: C. Gourlay and J. Jones) for its help in polishing our paper.
Funding: This work was supported by the Graduate Innovation Fund of Huazhong University of Science and Technology (2021yjsCXCY121).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-470
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-470
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-470). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the Union Hospital, Tongji Medical College of Huazhong University of Science and Technology (0304-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krishnan A, Berthelet J, Renaud E, et al. Proteogenomics analysis unveils a TFG-RET gene fusion and druggable targets in papillary thyroid carcinomas. Nat Commun 2020;11:2056. [Crossref] [PubMed]
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 2020;16:17-29. [Crossref] [PubMed]
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet 2016;388:2783-95. [Crossref] [PubMed]
- Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Coelho M, Raposo L, Goodfellow BJ, et al. The Potential of Metabolomics in the Diagnosis of Thyroid Cancer. Int J Mol Sci 2020;21:5272. [Crossref] [PubMed]
- Mulla MG, Knoefel WT, Gilbert J, et al. Lateral cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the lateral compartment. Clin Endocrinol (Oxf) 2012;77:126-31. [Crossref] [PubMed]
- Feng JW, Qin AC, Ye J, et al. Predictive Factors for Lateral Lymph Node Metastasis and Skip Metastasis in Papillary Thyroid Carcinoma. Endocr Pathol 2020;31:67-76. [Crossref] [PubMed]
- Yu J, Deng Y, Liu T, et al. Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat Commun 2020;11:4807. [Crossref] [PubMed]
- Yu XM, Lo CY, Chan WF, et al. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res 2005;11:8063-9. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Asare EA, Silva-Figueroa A, Hess KR, et al. Risk of Distant Metastasis in Parathyroid Carcinoma and Its Effect on Survival: A Retrospective Review from a High-Volume Center. Ann Surg Oncol 2019;26:3593-9. [Crossref] [PubMed]
- Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg 2018;53:1112-7. [Crossref] [PubMed]
- Baek S, Park SH, Won E, et al. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol 2015;16:286-96. [Crossref] [PubMed]
- Yao XI, Wang X, Speicher PJ, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst 2017; [Crossref] [PubMed]
- Zhang Z. Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med 2017;5:7. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Greifer N. Covariate Balance Tables and Plots: A Guide to the cobalt Package. 2021. Available online: https://cran.r-project.org/web/packages/cobalt/vignettes/cobalt.html
- Zhang M, Solomon DH, Desai RJ, et al. Assessment of Cardiovascular Risk in Older Patients With Gout Initiating Febuxostat Versus Allopurinol: Population-Based Cohort Study. Circulation 2018;138:1116-26. [Crossref] [PubMed]
- Geron Y, Benbassat C, Shteinshneider M, et al. Multifocality Is not an Independent Prognostic Factor in Papillary Thyroid Cancer: A Propensity Score-Matching Analysis. Thyroid 2019;29:513-22. [Crossref] [PubMed]
- Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci 2010;25:1-21. [Crossref] [PubMed]
- Rubin D. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Serv Outcomes Res Methodol 2001;2:169-88. [Crossref]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2018;68:55-63.
- Adam MA, Pura J, Goffredo P, et al. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol 2015;33:2370-5. [Crossref] [PubMed]
- Sippel RS, Robbins SE, Poehls JL, et al. A Randomized Controlled Clinical Trial: No Clear Benefit to Prophylactic Central Neck Dissection in Patients With Clinically Node Negative Papillary Thyroid Cancer. Ann Surg 2020;272:496-503. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Iida F, Yonekura M, Miyakawa M. Study of intraglandular dissemination of thyroid cancer. Cancer 1969;24:764-71. [Crossref] [PubMed]
- Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585-90. [Crossref] [PubMed]
- Russell WO, Ibanez ML, Clark RL, et al. Thyroid carcinoma. classification, intraglandular dissemination, and clinicopathological study based upon whole organ sections of 80 glands. Cancer 1963;16:1425-60. [Crossref] [PubMed]
- Black BM, Kirk TA Jr, Woolner LB. Multicentricity of papillary adenocarcinoma of the thyroid: influence on treatment. J Clin Endocrinol Metab 1960;20:130-5. [Crossref] [PubMed]
- Jin H, Yan H, Tang H, et al. Internal Spreading of Papillary Thyroid Carcinoma: A Case Report and Systemic Review. Case Rep Endocrinol 2018;2018:7618456. [Crossref] [PubMed]
- Shattuck TM, Westra WH, Ladenson PW, et al. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med 2005;352:2406-12. [Crossref] [PubMed]
- Lu Z, Sheng J, Zhang Y, et al. Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles. J Pathol 2016;239:72-83. [Crossref] [PubMed]
- Bansal M, Gandhi M, Ferris RL, et al. Molecular and histopathologic characteristics of multifocal papillary thyroid carcinoma. Am J Surg Pathol 2013;37:1586-91. [Crossref] [PubMed]
- Gerfo PL, Chabot J, Gazetas P. The intraoperative incidence of detectable bilateral and multicentric disease in papillary cancer of the thyroid. Surgery 1990;108:958-62; discussion 962-3. [PubMed]



