Robotic thyroidectomy and cervical neck dissection for thyroid cancer
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy (1,2). Although this type of thyroid cancer generally has an excellent prognosis, lymph node (LN) metastases are quite common. The reported incidence rate of clinical LN metastasis in patients with PTC is 30% at presentation (3); however, this rate increases to as high as 90% with routine cervical LN dissection and meticulous microscopic examination (4-8). Although the impact of regional LN metastases on long-term survival has long been controversial, LN metastasis is generally considered to increase the incidence of loco-regional recurrence even in low-risk patients (8,9). Given the excellent prognosis of PTC, loco-regional recurrence is a major concern that influences a patient’s quality of life (QOL) after thyroid surgery (9). Accordingly, the proper management of metastatic LNs is an essential component of surgery for thyroid cancer.
The conventional surgical treatment for thyroid cancer is open thyroidectomy; however, this method inevitably causes neck scarring because of the anatomical location of the thyroid gland. A cosmetic desire to avoid neck scars after thyroid surgery has led to the development of remote access endoscopic thyroidectomy (10,11). However, these endoscopic methods are technically challenging and require a long learning curve (12,13). Furthermore, cervical LN dissection, a standard procedure in the context of thyroid cancer surgery, is difficult to perform endoscopically (14-16).
The da Vinci robot system has improved endoscopic thyroid surgery by providing a three-dimensional magnified view, decreased tremor, and superior range of motion. These advantages are especially helpful when performing meticulous cervical LN dissection and have led to the consideration of robotic thyroid surgery as the best option for thyroid cancer surgery.
Compared with conventional open and endoscopic surgery, robotic thyroidectomy has provided excellent oncologic results with low complication rates when performed by experienced endocrine surgeons. Excellent cosmetic outcomes and reductions in pain and dysphagia have also been reported with this technique (17-21). Robotic thyroid surgery is particularly prevalent in Korea, where more than 8,000 cases of robotic thyroidectomy were performed from 2007 to 2011 (2,000 procedures in 2011 alone) (22).
Recently, the indication for robotic thyroid surgery has expanded to include more complicated procedures such as lateral neck dissection and modified radical neck dissection (MRND). Increasingly, surgeons are taking the initiative to perform robotic thyroidectomy with lateral neck dissection (23-25). In this article, we have described our robotic thyroidectomy and central/lateral neck node dissection procedures and have summarized the recent results associated with this surgical technique.
Surgical indications for robotic thyroidectomy
Initially, the indications for robotic thyroidectomy were limited to small thyroid tumors, including benign nodules and follicular neoplasms, and low-risk thyroid carcinomas without cervical LN metastasis (26,27). However, the surgical indications for robotic thyroidectomy have expanding as surgeons’ experience levels have increased. A recent web-based survey conducted by Bae et al. reported that the main indications for robotic thyroidectomy were papillary thyroid cancers <2 cm in size, intracapsular lesions, a non-posterior location, and no clinical evidence of lateral LN metastasis (22).
However, some surgeons have initiated the use of this robotic procedure for more difficult cases, such as those involving more advanced thyroid cancers (e.g., size >2 cm, extracapsular lesions, thyroid cancers with lateral LN metastasis) or Graves’ disease (23-25,28,29).
The following are recent surgical indications for robotic thyroidectomy at our institute:
- Benign thyroid nodule or follicular neoplasm not larger than 5 cm;
- Graves’ disease with a thyroid volume <150 mL;
- Thyroid cancer;
- Tumor size <4 cm;
- No significant tracheal or esophageal invasion;
- No great vessel invasion;
- Extrathyroidal invasion, central or lateral LN metastasis, and posterior location near the recurrent laryngeal nerve (RLN) are not contraindications.
Verticalizing maneuver (VM) in the bilateral axillo-breast approach (BABA) to robotic thyroid surgery
The two prevalent methods of robotic thyroid surgery are the trans-axillary approach (TAA) and the BABA (26,27). At our institute, we are using a general BABA method. Compared with the TAA, the BABA provides better cosmesis. Additionally, surgeons are more familiar with the midline approach used in the BABA. Therefore, ipsilateral and contralateral lobe resections are equally easy with the BABA; whereas in the TAA, contralateral lobe resection is more difficult (30). Contralateral lobectomy with TAA can be performed safely after a necessary learning curve. In a web-based survey conducted in South Korea in 2011, surgeons who used the BABA listed completeness of contralateral lobectomy and better cosmesis as advantages and difficulty with neck node dissection as a major disadvantage, whereas surgeons who used the TAA considered the facilitation of neck node dissection to be an advantage and difficulty with achieving a complete contralateral lobectomy as a disadvantage (22).
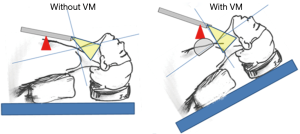
In the BABA, a camera shows a near-midline view from the caudal to the cephalic direction. However, because the distance between the surgical field and the viewpoint was short and the view angle was shallow in the original BABA, the operator could not gain sufficient access to the lower part of the neck near the clavicle for complete LN dissection. The use of an inflexible da Vinci camera and harmonic scalpel increased this challenge. Therefore, we have modified the positions of the patient and robotic arms through a VM (25,31). In brief, to remove the blind spot in the lower neck, patients are placed in a reverse-Trendelenburg position at 20º–30º, and the pivot point of the camera port is moved to an antero-superior direction (Figure 1). As a result, the patients’ neck and camera nearly form a right angle. The VM enables us to perform a more complete LN dissection that includes level VII in the central neck and levels IV and Vb in the lateral neck.
Surgical procedures
The surgical procedures used in the original BABA robotic thyroidectomy have been described in detail by Lee et al. (30). Earlier in our practice, we adopted the original BABA and later modified the original procedures used in this approach while treating more than 350 cases, including advanced cancers that required shaving of the RLN or bilateral lateral neck dissection. Herein, we describe our own modified BABA procedures.
Preparation
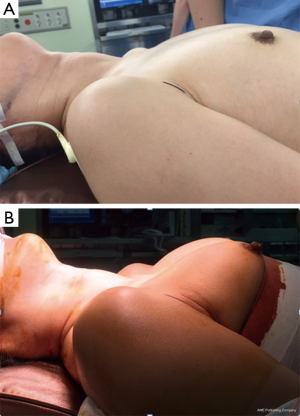
The patient position is the same as that used in the conventional open procedure. For VM, we encircle the lower portions of the breasts below the nipple with an elastic band to lift the circumareolar sites, which serve as the trocar pivot points (Figure 2). Aseptic draping is applied to expose the anterior neck, bilateral axilla, and bilateral areolar areas. The operating table is then placed in the reverse-Trendelenburg position to approximately 20º–30º (25,31).
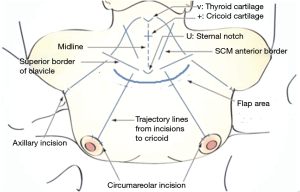
Some anatomical landmarks are marked, including the midline, thyroid cartilage notch, cricoid cartilage, medial borders of the bilateral sternocleidomastoid (SCM) muscles, superior borders of the bilateral clavicles, and four trajectory lines from each ipsilateral clavicle incision in the direction of the midportion of the cricoid cartilage (Figure 3). Approximately 100 cc of normal saline mixed with 3 mg/kg of 0.1% ropivacaine is then used to infiltrate the subcutaneous or subplatysmal layer of the flap dissection area with a 23-gauge spinal needle. We previously reported that infiltration of the flap sites with a ropivacaine-saline solution is a safe and effective method for reducing postoperative pain and postoperative analgesic consumption (32). Because the upper and mid-neck area flap is dissected sharply with robotic scissors, causing little pain, the pre-incisional injection is omitted in this area.
Skin incision and tunneling
A 12-mm-long right circumareolar incision is made for camera port, in addition to three additional 8-mm-long incisions (Figure 3). In male patients, the camera port and first arm trocar site are moved slightly upward from the circumareolar lesion because VM via circumareolar incisions is very difficult in male patients. These locations are approximately 7–10 cm below the clavicle, as the distance from the trocar tip to the pivot point is approximately 7 cm. After making the skin incisions, subcutaneous narrow trocar tunnels are carefully generated from the circumareolar incisions to just beyond the ipsilateral clavicles with straight mosquito forceps, followed by a vascular tunneler. A long Kelly hemostat may be used instead of a vascular tunneler. Next, a 12-mm camera port is inserted through the right breast incision, and an 8-mm port is inserted through the left breast incision. CO2 gas at a pressure of 8 mmHg insufflates the working space through the camera port. After making a small space in the lower neck area with ultrasonic shears, 8-mm axillary trocars are inserted along the axillary trajectory lines.
Robot docking and flap dissection
After the operating table is placed in the reverse-Trendelenburg position at a 20º–30º angle, robot docking is performed. The camera is inserted through the right breast, and the monopolare lectrocautery or ultrasonic shears is inserted through the left breast. For VM, the trocar pivot points should be as high as possible without much tension. For better visualization of each thyroid lobe, the camera port direction is set to the chin for right thyroidectomy and to the left mandibular angle for left thyroidectomy. For total thyroidectomy, the camera axis is changed after lesion-side lobectomy. Graspers (Prograsp forceps and Maryland forceps; Intuitive Surgical Inc., Sunnyvale, CA, USA) are inserted through the right and left axillary ports. Flap dissection, except for a small area near the sternal notch, is performed using robotic instruments after robot docking. The subfascial layer is preferred for flap dissection because it tends to cause fewer postoperative wound adhesions. For subfascial flap dissection, the anterior jugular veins should be ligated near the sternal notch, which can be done very safely using a bipolar coagulator connected with Maryland forceps, rather than ultrasonic shears. Flap dissection can also be performed safely and effectively with monopolar scissors, or hot shears.
Flap dissection is a very important step in robotic thyroid surgery for two reasons. First, this step influences the surgical stress and post-operative pain. From our prospective, randomized, and controlled trial, we learned that flap site pain, especially in the infra-clavicular and anterior chest area, was the most important pain after robot thyroid surgery (32). Vigorous blunt dissection with a vascular tunneler may cause serious bleeding and postoperative pain. In the original BABA, flap dissection began by elevating the anterior chest flap with a vascular tunneler, followed by extension with ultrasonic shears from the thyroid cartilage superiorly to 2 cm below the clavicle inferiorly. To lessen postoperative flap site pain and paresthesia, we needed to reduce the flap dissection area. VM allows a more vertical view of the surgical field, thus omitting the need for flap dissection in the infra-clavicular and anterior chest area. Instead, we use a vascular tunneler only to make narrow tracks for trocar insertion. Second, considerable complications, such as flap burns or perforations, may occur inadvertently during this step. Because cosmesis is a major motivation for robotic thyroid surgery, these complications are troublesome for both the patient and the surgeon. We use robotic hot shears under a robotic setting instead of the ultrasonic shears generally used for flap dissection under an endoscopic setting. We can easily identify an avascular flap plane under three-dimensional vision and can perform sharp dissection with scissors under traction and counter-traction from the other two robotic arms. We believe that this method not only helps to generate a clean flap without bleeding, but also reduces thermal damage and flap complications.
Midline division
Robotic BABA procedures for thyroidectomy are very similar to those for conventional open thyroidectomy, except for the use of robotic instruments under three-dimensional camera vision. The BABA begins with midline division of the strap muscle, whereas the TAA uses a lateral approach. Herein, the isthmus is divided, the midline division is extended, and dissection is extended from the suprasternal notch inferiorly to the thyroid cartilage notch superiorly via monopolar electrocautery as in conventional open surgery.
Dissection of the pyramidal lobe and pre-laryngeal LN
Occasionally, the pyramidal lobe is extended upwardly to the hyoid bone, and a thyroglossal duct cyst is incidentally found. These should be removed completely to ensure the complete removal of thyroid tissue. The delphian or prelaryngeal nodes may be included in the soft tissue between the bilateral cricothyroid muscles. This soft tissue is routinely removed together with the thyroid tissue when LN dissection is necessary. This area always includes small vessels that can be controlled with monopolar electrocautery. Attention should be paid not to injure the cricothyroid muscle.
Division of the isthmus
Occasionally, preoperative ultrasonography reveals thyroid nodules in the isthmus. Division of the isthmus should be performed while avoiding penetration of the nodules. The upper border of the isthmus always contains large vessels that should be coagulated carefully.
Detachment from the strap muscle on the lateral side
This can be facilitated by medially retracting the thyroid lobe with Prograsp forceps and laterally retracting the strap muscle with Maryland forceps.
Detachment from the trachea and the cricoid cartilage
Dissection of the medial side facilitates thyroid surgery. The capsular plane should be identified in the safe inferior pole area, and capsular dissection should be continued as far as possible on the medial side. The thyroid tissue should never be entered or penetrated during this step to avoid injury of the RLN.
Dissection of the upper pole
Superior thyroidal vessels can be cauterized and divided with ultrasonic shears or via bipolar electrosurgery connected to Maryland forceps. These vessels should be divided into branches distal to the cricothyroid muscle so as not to injure the external branch of the superior laryngeal nerve. In most cases, a posterior branch of the superior thyroidal vessels, which supplies the superior parathyroid gland (PTG), can be preserved with a meticulous capsular dissection. Occasionally, and especially in goiters, the superior pole may extend to a very high position and adhere to the inferior pharyngeal constrictor muscle. Even in such cases, the three-dimensional magnified view and a meticulous capsular dissection allow us to remove almost all thyroid parenchyma without injuring the inferior pharyngeal constrictor muscle or the external branch of the superior laryngeal nerve.
Dissection of the lower pole
We attempt to find a capsular plane on the medial side of the lower pole and proceed with dissection toward the lateral side while maintaining the capsular plane.
Preservation of the RLN and PTG
A meticulous capsular dissection is essential for preservation of the RLN and PTG. The operator can observe the exact relationship between the PTGs and their feeding vessels only after all false capsules covering thyroid gland have been removed. We attempt to preserve all possible feeding vessels. The RLN is usually encountered near the Berry ligament during capsular dissection. Near the Berry ligament, careful dissection is needed to avoid any traction or thermal injury of the RLN. Intraoperative neural monitoring helps to find and preserve the RLN. The completely detached thyroid lobe is removed through the left axillary incision with an endoplastic bag (EndoCatch, Auto Suture; United States Surgical, Norwalk, CT, USA).
Central LN dissection
We prefer to perform central LN dissection separately from thyroid gland resection, unless metastatic LNs have adhered to the gland. We believe that capsular dissection, or dissection of the thyroid gland with little attached tissue, is advantageous for preservation of the RLN and PTGs. As noted above, VM is very helpful for reaching deep-seated LNs. To apply the VM, the pivot points of the robotic arms are relocated as high as possible without causing significant tension on the skin flap. The use of hot shears instead of a monopolar hook or ultrasonic shears is also very helpful. The hot shears has articulations and can coagulate, cut, and dissect the tissue.
For complete and safe central LN dissection, surgeons should understand the anatomical relationship between the thymic remnants, inferior PTG, and soft tissues that contain LNs. The size and visibility of the thymic remnants vary. One or two vertical inferior thyroidal veins always run along the thymus and can help to identify the dissection plane. The central compartment LNs are located deep to the plane of these veins and the thymus. The inferior PTG is always located in the superficial plane, usually near or within the thymic remnant. Preservation of the thymus has the advantage of decreasing the risk of transient and permanent hypoparathyroidism. Central LNs separated from the superficial thymus and inferior PTG are swept to the medial side from the common carotid artery. The RLN should be identified and preserved during this step; the use of a nerve stimulator connected to hot shears is helpful. Although this is unlikely on the left side, some LNs are located deep to the RLN on the right side, and these para-esophageal LNs should be resected in cases of right-sided advanced cancer.
Lateral neck LN dissection; MRND (Figure 4)
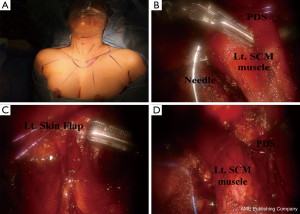
The procedures associated with robotic lateral neck dissection via the BABA are basically similar to those associated with the open surgical procedure and have been described in detail elsewhere (25); this procedure requires a larger skin flap that extends up to the inferior border of the submandibular gland, superiorly to the angle of the mandible, and posteriorly to the anterior edge of the trapezius. Fascia between the sternohyoid and SCM muscles is incised. After the medial and lateral borders of the SCM muscle are fully exposed, the SCM muscle is pulled with anchoring #0 polydioxanone sutures [polydioxanone suture (PDS); Ethicon, San Angelo, TX, USA]. Inferior level IV dissection is performed while preserving the transverse cervical artery and phrenic nerve. The branches of the thoracic duct are ligated using robotic Hem-o-Lock clips (Weck Surgical Instrument; Teleflex Medical, Durham, NC, USA). Level Vb dissection is performed while preserving the spinal accessory nerve. Level II dissection is advanced until the posterior belly of the digastric muscle is exposed superiorly, preserving the spinal accessory nerve. It is helpful to reposition the direction of the camera port so that it corresponds with the level of dissection. A slight clockwise or counterclockwise rotation of the camera port is needed.
Closure
After irrigation and meticulous hemostasis, midline closure is achieved by running sutures. A suction drain is reserved only for lateral neck dissection. The skin incisions are closed with absorbable sutures.
Robotic central and lateral neck LN dissection
Central neck dissection (CND) for the treatment of PTC is needed in patients with advanced primary disease (33-35). Although the impact of CND on long-term survival has been controversial, some centers including ours even routinely perform prophylactic CND in all PTC patients (35,36). However, CND in the context of endoscopic or robotic thyroidectomy is technically challenging (31). The harmonic scalpel has a limited range of motion and lacks side-bending capabilities. In addition, the camera is not flexible and the surgical view is limited. With VM, we can perform a more complete LN dissection that includes level VII in the central neck and levels IV and Vb in the lateral neck. In addition, we currently use a hot shears, which has side-bending capabilities and provide an enhanced range of motion, instead of a monopolar hook and harmonic scalpel. The use of this sharp instrument enables more delicate manipulation.
The clinical parameters used to evaluate surgical completeness after robotic CND include the number of retrieved LNs and stimulated TG levels. In most studies, the numbers of retrieved central LNs and stimulated TG levels in the robotic thyroidectomy group were comparable to those in the open thyroidectomy group without sacrificing the surgical safety parameters such as hypoparathyroidism and RLN injury rates (14,15,18,19,28,37,38). However, in some studies, including our previous study, the absolute number of retrieved LNs was lower in patients undergoing robotic thyroidectomy (31,39). Additionally, studies that compared robotic thyroidectomy and endoscopic thyroidectomy groups reported higher numbers of retrieved LNs in the robotic thyroidectomy groups (14,15) (Table 1).
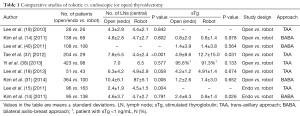
Full table
PTC patients with lateral cervical LN metastases must undergo MRND (13,33,41). The American Thyroid Association guideline recommends that patients with DTC and lateral cervical LN metastases undergo comprehensive neck dissection of levels II-V because of the high rate of locoregional recurrence (up to 30%) and the possibility of skip metastasis (33). The conventional open method with a long cervical incision has been adopted as the treatment of choice worldwide (42). Although the length of the skin incision has been reduced with increasing experience, a long, prominent neck incision scar is inevitable (43).
Robotic MRND may be a good alternative option for avoiding a long neck scar. Both the TAA and BABA have applied for robotic MRND and have yielded comparable outcomes to conventional open surgery. A comparison of patients undergoing TAA- or BABA-based robotic MRND with those undergoing open surgery reported similar surgical completeness rates and short-term recurrence rates, without any surgical complications such as hypocalcemia, RLN paralysis, chyle leak, and bleeding (18,25,28) (Table 2). However, robotic lateral neck dissection remains at an early stage for some initiative surgeons, and further evaluations are required.
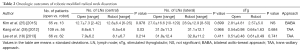
Full table
The BABA is a multidirectional approach in which the midline is the viewpoint, whereas the TAA is a lateral approach. The symmetry of the BABA system can enable bilateral lateral neck dissection. We have recently succeeded in performing a robotic bilateral MRND (ongoing publication process). During this bilateral MRND procedure, the camera was moved from the right breast trocar site to the left breast trocar site, which enabled dissection of the contralateral lateral neck.
Limitations
The first limitation of robotic thyroidectomy is the loss of tactile feedback (43). A fine manual sense cannot be accomplished with robotic instruments. However, most surgeons may compensate for this drawback by using magnified three-dimensional visual feedback. The second limitation is the medical cost (44). Da Vinci robotic systems may be affordable only for some publicly funded hospitals or private hospitals with certain patient volumes.
Technical advances are needed to overcome the current limitations of robotic thyroid surgery. Additionally, guidelines are needed to reduce trial errors. Other challenges include the expense associated with robotic instruments and the delayed acceptance of new technology by old surgeons who are skilled in conventional open technologies (43).
Conclusions
The oncologic outcomes of robotic thyroidectomy with cervical neck dissection are comparable to those of open procedures, without sacrificing surgical safety parameters. Application of the da Vinci robot in thyroid cancer surgery allows meticulous cervical LN dissection. The use of this robot for cervical neck dissection will continue to evolve, and the indications for robotic thyroidectomy will continue to expand. Prospective, randomized, comparative clinical trials using validated methods and including long-term follow-ups are needed to further validate the existing results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1-14. [Crossref] [PubMed]
- Shaha AR, Shah JP, Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg 1996;172:692-4. [Crossref] [PubMed]
- Dixon E, McKinnon JG, Pasieka JL. Feasibility of sentinel lymph node biopsy and lymphatic mapping in nodular thyroid neoplasms. World J Surg 2000;24:1396-401. [Crossref] [PubMed]
- Dzodic R, Markovic I, Inic M, et al. Sentinel lymph node biopsy may be used to support the decision to perform modified radical neck dissection in differentiated thyroid carcinoma. World J Surg 2006;30:841-6. [Crossref] [PubMed]
- Kelemen PR, Van Herle AJ, Giuliano AE. Sentinel lymphadenectomy in thyroid malignant neoplasms. Arch Surg 1998;133:288-92. [Crossref] [PubMed]
- Pelizzo MR, Boschin IM, Toniato A, et al. The sentinel node procedure with Patent Blue V dye in the surgical treatment of papillary thyroid carcinoma. Acta Otolaryngol 2001;121:421-4. [Crossref] [PubMed]
- Sakorafas GH, Sampanis D, Safioleas M. Cervical lymph node dissection in papillary thyroid cancer: current trends, persisting controversies, and unclarified uncertainties. Surg Oncol 2010;19:e57-70. [Crossref] [PubMed]
- Machens A, Hinze R, Thomusch O, et al. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 2002;26:22-8. [Crossref] [PubMed]
- Ikeda Y, Takami H, Niimi M, et al. Endoscopic thyroidectomy by the axillary approach. Surg Endosc 2001;15:1362-4. [Crossref] [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 2007;31:601-6. [Crossref] [PubMed]
- Lee J, Yun JH, Nam KH, et al. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol 2011;18:226-32. [Crossref] [PubMed]
- Lee J, Yun JH, Choi UJ, et al. Robotic versus Endoscopic Thyroidectomy for Thyroid Cancers: A Multi-Institutional Analysis of Early Postoperative Outcomes and Surgical Learning Curves. J Oncol 2012;2012:734541.
- Kim WW, Kim JS, Hur SM, et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J Surg 2011;35:779-84. [Crossref] [PubMed]
- Lee J, Lee JH, Nah KY, et al. Comparison of endoscopic and robotic thyroidectomy. Ann Surg Oncol 2011;18:1439-46. [Crossref] [PubMed]
- Lee S, Ryu HR, Park JH, et al. Excellence in robotic thyroid surgery: a comparative study of robot-assisted versus conventional endoscopic thyroidectomy in papillary thyroid microcarcinoma patients. Ann Surg 2011;253:1060-6. [Crossref] [PubMed]
- Bae DS, Woo JW, Paek SH, et al. Antiadhesive effect and safety of sodium hyaluronate-carboxymethyl cellulose membrane in thyroid surgery. J Korean Surg Soc 2013;85:199-204. [Crossref] [PubMed]
- Lee J, Kwon IS, Bae EH, et al. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab 2013;98:2701-8. [Crossref] [PubMed]
- Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. [Crossref] [PubMed]
- Patel D, Kebebew E. Pros and cons of robotic transaxillary thyroidectomy. Thyroid 2012;22:984-5. [Crossref] [PubMed]
- Sun GH, Peress L, Pynnonen MA. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol Head Neck Surg 2014;150:520-32. [Crossref] [PubMed]
- Bae DS. Current status of robotic thyroid surgery in South Korea: a web-based survey. World J Surg 2014;38:2632-9. [Crossref] [PubMed]
- Tae K, Ji YB, Song CM, et al. Robotic lateral neck dissection by a gasless unilateral axillobreast approach for differentiated thyroid carcinoma: our early experience. Surg Laparosc Endosc Percutan Tech 2014;24:e128-32. [Crossref] [PubMed]
- Kang SW, Lee SH, Ryu HR, et al. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010;148:1214-21. [Crossref] [PubMed]
- Seup Kim B, Kang KH, Park SJ. Robotic modified radical neck dissection by bilateral axillary breast approach for papillary thyroid carcinoma with lateral neck metastasis. Head Neck 2015;37:37-45. [Crossref] [PubMed]
- Lee KE. Outcomes of 109 patients with papillary thyroid carcinoma who underwent robotic total thyroidectomy with central node dissection via the bilateral axillo-breast approach. Surgery 2010;148:1207-13. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [Crossref] [PubMed]
- Kang SW, Lee SH, Park JH, et al. A comparative study of the surgical outcomes of robotic and conventional open modified radical neck dissection for papillary thyroid carcinoma with lateral neck node metastasis. Surg Endosc 2012;26:3251-7. [Crossref] [PubMed]
- Kwon H. Bilateral axillo-breast approach robotic thyroidectomy for Graves' disease: an initial experience in a single institute. World J Surg 2013;37:1576-81. [Crossref] [PubMed]
- Lee KE, Choi JY, Youn YK. Bilateral axillo-breast approach robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 2011;21:230-6. [Crossref] [PubMed]
- Kim BS, Kang KH, Kang H, et al. Central neck dissection using a bilateral axillo-breast approach for robotic thyroidectomy: comparison with conventional open procedure after propensity score matching. Surg Laparosc Endosc Percutan Tech 2014;24:67-72. [Crossref] [PubMed]
- Kang KH, Kim BS, Kang H. The benefits of preincision ropivacaine infiltration for reducing postoperative pain after robotic bilateral axillo-breast approach thyroidectomy: a prospective, randomized, double-blind, placebo-controlled study. Ann Surg Treat Res 2015;88:193-9. [Crossref] [PubMed]
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg 2006;30:91-9. [Crossref] [PubMed]
- Sancho JJ, Lennard TW, Paunovic I, et al. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 2014;399:155-63. [Crossref] [PubMed]
- Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab 2009;94:1162-7. [Crossref] [PubMed]
- Tae K, Ji YB, Cho SH, et al. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years' experience. Head Neck 2012;34:617-25. [Crossref] [PubMed]
- Yi O, Yoon JH, Lee YM, et al. Technical and oncologic safety of robotic thyroid surgery. Ann Surg Oncol 2013;20:1927-33. [Crossref] [PubMed]
- Tae K, Ji YB, Jeong JH, et al. Comparative study of robotic versus endoscopic thyroidectomy by a gasless unilateral axillo-breast or axillary approach. Head Neck 2013;35:477-84. [Crossref] [PubMed]
- Lee KE. Surgical completeness of bilateral axillo-breast approach robotic thyroidectomy: comparison with conventional open thyroidectomy after propensity score matching. Surgery 2011;150:1266-74. [Crossref] [PubMed]
- Machens A, Hauptmann S, Dralle H. Lymph node dissection in the lateral neck for completion in central node-positive papillary thyroid cancer. Surgery 2009;145:176-81. [Crossref] [PubMed]
- Silver CE, Rinaldo A, Ferlito A. Crile's neck dissection. Laryngoscope 2007;117:1974-7. [Crossref] [PubMed]
- Lee J, Chung WY. Current status of robotic thyroidectomy and neck dissection using a gasless transaxillary approach. Curr Opin Oncol 2012;24:7-15. [Crossref] [PubMed]
- Inabnet WB 3rd. Robotic thyroidectomy: must we drive a luxury sedan to arrive at our destination safely? Thyroid 2012;22:988-90. [Crossref] [PubMed]