
The incidence of postoperative re-stratification for recurrence in well-differentiated thyroid cancer—a retrospective cohort study
Introduction
The past 20 years have witnessed many advances in the diagnosis and treatment of thyroid nodules as well as well-differentiated thyroid cancer (WDTC), but controversies in many clinical areas remain unresolved. The extent of thyroidectomy for WDTC continues to be one of the main subjects of debate. One large population-based cohort analysis of large databases [Surveillance, Epidemiology and End Results (SEER) & Nationwide Inpatient Sample (NIS)] showed that papillary thyroid carcinoma (PTC) has a good overall prognosis, with the extent of surgical resection making no significant difference in overall survival (OS) or disease-specific survival in tumors <4 cm (1,2). Factors that have been considered to decrease disease-specific survival and OS include advanced age, increased tumor size, extrathyroidal tumor growth, and regional and distant metastases (1-4). The American Thyroid Association (ATA) 2015 guidelines encourage careful preoperative risk assessment to avoid unnecessary major surgery and to reduce the risk of surgical complications without increasing risk of recurrence and metastasis (5). A major goal of these guidelines is to minimize potential harm from overtreatment in a majority of patients who are at low risk for disease-specific recurrence, while appropriately treating and monitoring those patients at higher risk.
The ATA recommends initial risk assessment that classifies patients with 1–4 cm WDTC and free of suspicious features {i.e., nodal involvement (cN0), invasion to adjacent structures [without extrathyroidal extension (ETE)], aggressive histology} as being suitable candidates for hemi-thyroidectomy (HT), with less risk of complications and only a slightly higher risk of locoregional recurrence (5). Some characteristics can be determined before or during a thyroidectomy. These include evidence of gross ETE, locoregional or distant metastases, and a history of radiation or a positive family history. Patients known to have these characteristics are preoperatively classified as being at high-risk for disease-specific recurrence and they are generally recommended to undergo at least total thyroidectomy (TT), which also enables radioiodine adjuvant treatment. The other high-risk characteristics, however, will only become apparent upon histopathological examination in the postoperative setting (i.e., microscopic ETE, positive tumor margins, incidental positive lymph nodes in the specimen, vascular invasion, and an aggressive tumor subtype). Therefore, patients are re-stratified according to intra- and postoperative findings as well as histopathological features after which, if indicated, completion thyroidectomy is recommended.
The aim of our study was to assess the incidence of significant high-risk features for disease-specific recurrence that were determined postoperatively among the WDTC patients operated in our institution who had been considered preoperatively as being at low risk. We also compared the preoperative characteristics of the patients who were upscaled to a higher risk level postoperatively with those who maintained their preoperative risk level.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-105).
Methods
Patients
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of the Tel-Aviv Sourasky Medical Center (TLV-704-16) and individual consent for this retrospective analysis was waived. The work has been reported in line with the STROCSS criteria (6). All patients who underwent thyroid surgery (HT and TT) between 2006–2018 at Tel-Aviv Sourasky Medical Center (TASMC) were included. Their medical charts were reviewed, and the data on demographics, clinical presentation and workup, intraoperative report, and pathological report were retrieved. The patients were stratified preoperatively and postoperatively according to the ATA 2015 guidelines (5). They all underwent an ultrasound (US) examination of the head and neck and fine needle aspiration of the thyroid lesion prior to surgery.
Risk stratification
Patients were defined as being at low risk for disease-specific recurrence if they had well-differentiated thyroid tumors between 1–4 cm in size, free of any evidence of positive cervical lymph nodes, invasion to adjacent structures, and high-risk cytology. Clinically positive nodes were defined as being abnormal by US findings. We excluded patients not eligible for initial HT according to the National Comprehensive Cancer Network (NCCN) and the ATA thyroid cancer management guidelines. Thus, patients with thyroid tumors that were not well-differentiated, or with well-differentiated thyroid tumors that were smaller than 1 cm or larger than 4 cm in size, were excluded from the study. Other exclusion criteria included patients with other malignancies except for the thyroid malignancy, and patients with preoperatively known high risk characteristics, according to preoperatively imaging and clinical examination, such as gross extra-thyroidal extension on preoperative imaging, clinically apparent cervical lymph node metastases, distant metastases, vocal cord paralysis or immobility on physical examination, history of radiation and positive family history (Figure 1).
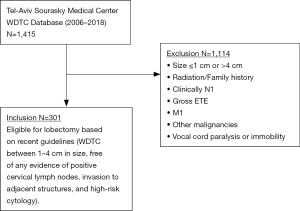
None of the patients included had undergone a prophylactic central lymph node dissection.
Patients who had been classified as low-risk before surgery were escalated in risk stratification and re-classified after surgery as intermediate-to-high risk if gross extra-thyroidal extension had been observed intraoperatively with concordant microscopic extra-thyroidal extension in final histopathology, if the final histopathology results showed aggressive histology (e.g., tall cell, diffuse sclerosing variant), perineural (7-9) or lymphovascular invasion, if there were more than 5 positive involved lymph nodes (larger than 0.2 cm) within the specimen, or if extra-nodal extension, incomplete tumor resection (positive margins) or distant metastases had been detected during or after surgery (5).
Statistical analysis
Continuous variables were evaluated for normal distribution by means of a histogram and a Q-Q plot and reported as mean and standard deviation (normally distributed variables) or median and interquartile range (skewed variables). Categorical variables were reported as frequency and percentage. The chi-squared test or Fisher’s exact test were used to compare categorical variables between the two groups of patients, and independent samples t-test or Mann-Whitney test were employed to compare the continuous variables. A P value of <0.05 was considered statistically significant. All statistical analyses were by SPSS (IBM SPSS Statistics for Windows, version 25, IBM Corp., Armonk, NY, USA, 2017).
Results
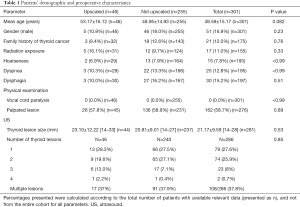
The medical charts of 1,415 patients who underwent thyroid surgery between January 2006 and December 2018 in TASMC were retrieved. Three hundred and one patients have met the inclusion criteria and were stratified as being at low-risk for disease-specific recurrence. Table 1 summarizes the patients’ demographic and pre-operative characteristics (symptoms, physical examination, and sonographic findings). Most of them (n=250, 83.1%) were females. The mean age of the entire cohort was 49.59±15.17 years (median 50 years, interquartile range 38–61 years). Of note, the presenting symptoms reported by some patients (such as hoarseness, dyspnea and dysphagia) are unusual in patients with low risk WDTC. However, our cohort included patients who were admitted at our department for surgery and were asked specifically regarding the initial complaints that have led them to medical consultation. Thus, any subjective complaint they reported was documented, even if there was no objective explanation for the cause of these symptoms. Also, important to mention, that those reported symptoms were mild, with no supporting findings in the physical examination.
Full table
Forty-six patients were upscaled postoperatively from low risk to an intermediate-to-high risk level (see “Methods” section) yielding a rate of postoperative risk escalation of 15%. The other 255 (84.7%) patients remained at their preoperative low-risk level. The 46 patients that were upscaled by the risk stratification for recurrence scale postoperatively, were re-classified due to the following causes: 3 (6.5%) had perineural invasion, 15 (32.6%) had vascular invasion, and 26 (56.5%) had documented intra-operative clinical impression of gross ETE followed by histological confirmation. The remaining 2 patients were upscaled due to lymph node characteristics: one patient had extra-nodal extension and the other had >5 metastatic lymph nodes larger than 0.2 cm that were resected from a suspected firm conglomerate during surgery.
All the patients underwent a preoperative US examination that demonstrated a dominant lesion with a mean size of 21.17±9.59 mm and a median size of 19 mm. One hundred and eight patients (35.8%) had multiple thyroidal lesions. There were no significant differences in the demographic characteristics, medical history, initial clinical presentation, physical examination findings upon presentation, or sonographic characteristics between the patients that were upscaled postoperatively to those whose low-risk status stayed the same.
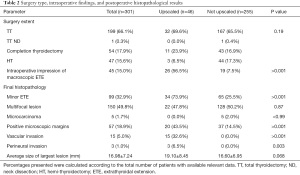
Table 2 summarizes the types of surgery, the intraoperative findings, and the histopathological results of the patients in our cohort. Of note, the majority of our patients were operated prior to the most recent ATA 2015 guidelines. Thus, although all of the 255 patients that were classified as low-risk pre- and postoperatively were probably eligible for lobectomy only nowadays, according to the ATA 2015 guidelines (5), only 44 (17.3%) patients of those 255 underwent HT without completion, while the rest underwent TT or completion after the initial HT, according to the ATA guidelines that were relevant at the operation time of each patient.
Full table
The histopathological analysis determined that the average lesion size was 16.98±7.24 mm, and that minor ETE was present in 99 cases (32.9%). One hundred and fifty patients (49.8%) had multifocality. Only 5 cases (1.7%) were defined as microcarcinomas (<10 mm final lesion size). The microscopic margins were positive for disease in 57 patients (18.9%). Three patients (1.0%) were found to have perineural invasion, and 15 patients (5.0%) were found to have vascular invasion.
When we compared both groups for their intra-operative course and final histopathological characteristics, perineural and vascular invasion as well as gross intra operative ETE were defined as criteria for upscaling (see “Methods” section). Therefore, these were significantly different between both groups. Except for these defining criteria, no significant differences were found in the other operative and histopathological characteristics (Table 2).
Regarding the central neck compartment, important to mention that none of the patients included had undergone a prophylactic central lymph node dissection. Since we have excluded patients who underwent neck dissection, all lymph nodes detected in the 301 patients’ cohort, were found incidentally on histopathologic examination. Six of those 301 patients (2.0%) had incidental lymph nodes detected in their final histopathology specimens. None of the patients had more than 5 positive lymph nodes removed. Of these, only in 1 patient (0.3%), a single positive microscopic lymph was detected. This finding didn’t alter the patient’s risk stratification. Moreover, none of these six patients with incidental lymph nodes detected in their final histopathology specimens was upscaled regarding risk stratification, thus none of them was included in the 46 upscaled patients’ cohort. The eighth AJCC edition (www.cancerstaging.org) defined the age factor as critical in determining the pre-operative risk stratification of WDTC (7,8).
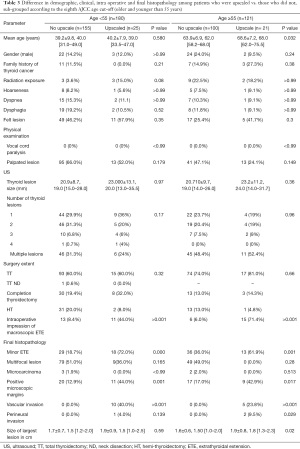
Accordingly, we further analyzed our data according to different age groups (Table 3). One hundred and twenty-one (40.2%) patients were older than 55 years of age. The microscopic positive margins were found to be significantly more common in the upscaled specimens of both younger and older groups [20 (12.9%) vs. 11 (44.0%), P=0.001; 17 (17.0%) vs. 9 (42.9%), P=0.007; accordingly]. Interestingly in the older age group both patient age (63.9±6.9 vs. 68.6±7.2, P=0.032) and tumor size (1.5±0.64 vs. 1.9±0.7) were found to be predictors of tumor upscaling.
Full table
In order to clarify the effect of the ATA 2015 guidelines (published in 2016) on the amount and characteristics of upscaled patients, we further divided our cohort into patients upscaled prior to the publication of the 2015 ATA guidelines, and those upscaled after the publication (Table 4).
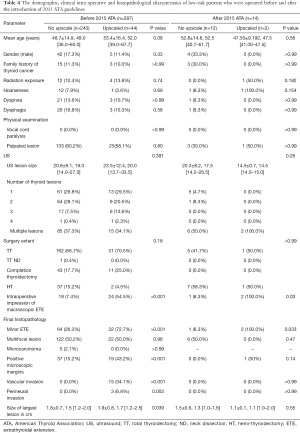
Full table
The surgeries’ complications in our cohort were as follows: 1 patient (0.3%) had dehiscence of the surgical scar that resolved spontaneously by secondary healing. Ten patient (3.2%) had transient hypocalcemia. Injury to the recurrent laryngeal nerve occurred in 7 patients (4.6%)—5 patients (1.7%) had transient unilateral vocal fold impairment, and 2 patient (0.7%) had permanent unilateral vocal fold paralysis.
Discussion
Preoperative estimation of the level of risk for disease-specific recurrence in WDTC is essential to determine whether to perform HT or TT. The incidence and clinical implications of high-risk features discovered postoperatively in patients with preoperatively determined low-risk WDTC are yet to be better defined (9-11).
In this study, we reviewed all patients who underwent thyroid surgery between 2006 and 2018 at TASMC. In total, 255 (84.7%) patients retained their preoperative low-risk level and thus were eligible for lobectomy according to the ATA 2015 guidelines. Forty-six patients were upscaled postoperatively from low risk to an intermediate-to-high risk level yielding a rate of postoperative risk escalation of 15%.
Shah et al.’s review of a consecutive series of 931 previously untreated patients with WDTC treated over a 50-year period showed OS of 87% at 10 years (3). Favorable prognostic factors included female gender, multifocal primary tumors, and regional lymph node metastases. Adverse prognostic factors included age over 45 years, follicular histology, ETE, tumor size >4 cm, and the presence of distant metastases. On multivariate analysis, the only factors that affected the prognosis were age, histology, tumor size, ETE, and distant metastases (3). These observations support the findings of reports from the Mayo Clinic and the Lahey Clinic on the significance of prognostic factors for WDTC (3).
In October 2016, the AJCC (www.cancerstaging.org) published the eighth edition of the AJCC/TNM cancer staging system, revising thyroid cancer staging. Among the modifications made, was an increase of the age cutoff from 45 to 55 years of age at diagnosis and including age as a major factor determining prognostic stage groups and the risk of WDTC preoperatively (7,8). Accordingly, we performed a subgroup analysis to the two age groups (younger and older than 55 years of age) comparing low risk patients who were upscaled to higher risk group, to those who were not upscaled. Interestingly, only in the older age group patients’ age and tumor size were predictors of upscaling. Thus, we recommend considering a more aggressive approach towards this subgroup, discussing the possible risks of partial thyroidectomy in the presence of a large tumor in an older patient (Table 3).
Historically, TT has been the operative treatment of choice for WDTC larger than 1 cm. Nixon et al. (4) showed that HT is a safe alternative to TT for T1/T2 N0 WDTC. Their practice for T1/T2 N0 WDTC is to have an informed discussion with the patient about the options of HT vs. TT. If the thyroid mass is a single nodule <4 cm with no nodules in the opposite lobe and no evidence of ETE, the patients are given the option of undergoing a HT. They do not recommend central compartment neck dissection unless there are palpable nodes at the time of surgery or if abnormal nodes are detected by preoperative ultrasonography.
Although both operations are considered safe, particularly when performed by a high-volume surgeon or in a high-volume center, it is well known that unlike HT, TT is associated with increased operative risk and surgical morbidity [particularly related to postoperative hypoparathyroidism and the rare complication of bilateral recurrent laryngeal nerve injury requiring tracheostomy (12)], increased total hospital charges, and longer hospital stay (1). The preservation of one thyroid lobe in HT may also obviate the need for thyroid hormone replacement (13).
Despite the advantages of HT compared to TT and the observational studies suggesting very low progression rates for WDTC (1-5), some studies have demonstrated that TT may be superior to HT, due to a lower rate of local recurrence following TT (14-16).
TT also allows for the use of radioactive iodine (RAI) in both postoperative treatment and surveillance. In their systematic review of recurrence rate and survival after HT for low-risk WDTC, Chan et al. (17) identified 31 studies (with a total of 228,746 patients (HT: 36,129, TT: 192,617), which had published recurrence and/or survival data for patients having had HT for WDTC. Pooled recurrence rates were 9.0% for HT (which is significantly higher than in previously published reports) compared to 7.4% for TT, [odds ratio (OR), 1.45; confidence interval (CI): 1.16–1.81, P=0.001]. Further, this rate was maintained when examining patients within low-risk cohorts established with recognized risk classifications (AGES, MACIS, AMES, AJCC). Subgroup analysis demonstrated a pooled recurrence rate of 9.2% for HT and 5.3% for TT. They also discovered that of those patients who develop recurrent disease, 48% recur outside the central neck. Pooled 10-year OS rates were similar—95.7% for HT and 95.8% for TT (OR, 0.92; CI: 0.73–1.18, P=0.52), consistent with current opinion that OS in low-risk WDTC is favorable independent of surgical extent.
Although their findings indicate that there is a small but significantly higher recurrence rate after HT compared to TT, the evidence base was heterogeneous and subject to confounding factors and would ultimately benefit from prospective randomized trials to overcome these deficiencies.
Several recent studies showed no significant difference in both disease-free survival and recurrence rate in patients with WDTC >1 cm after HT vs. TT (2,4). Based on these studies, the most recent NCCN and ATA guidelines support the option of HT for patients without preoperatively known high risk characteristics. Another factor that affects prognosis and should be taken into consideration when deciding on the extent of surgery is the histologic subtype of papillary thyroid cancer. In their study, Rajjoub et al. (18) aimed to examine whether survival is affected by extent of surgery for conventional vs. follicular-variant papillary thyroid cancer when stratified by tumor size. They evaluated 33,816 adults undergoing surgery for papillary thyroid cancer from 2004 to 2008 for 1.0–3.9 cm tumors and clinically negative lymph nodes. A total of 30,981 patients had TT and 2,835 had HT; 22,899 patients had conventional papillary thyroid cancer and 10,918 had follicular-variant papillary thyroid cancer. TT was associated with improved survival for conventional (P=0.02) but not for follicular-variant papillary thyroid cancer patients (P=0.42). For conventional papillary thyroid cancer, adjusted analysis showed TT was associated with improved survival for 2.0–3.9 cm tumors (P=0.03) but not for 1.0–1.9 cm tumors (P=0.16). For follicular-variant, HT and TT had equivalent survival for 1.0–1.9 cm (P=0.45) and 2.0–3.9 cm (P=0.88) tumors.
Rarely are the cases that intraoperatively the surgeon encounters surprising findings that mandate extreme change of the preoperative plan. It is important to mention that even one incidental positive lymph node in the specimen after a procedure without formal central compartment neck dissection implies the high possibility that some microscopic nodal metastases have been left behind and may lead to the upscaling the patient risk status. However, the clinical significance of a single microscopic lymph node is yet to be determined. Randolph et al. (19) showed that the risk of recurrence in small-volume microscopic N1 subclinical disease is much reduced than in large-volume, macroscopic clinically apparent loco-regional metastases. According to their study (19), in patients with histopathological proven lymph node metastases (pN1), the risk of locoregional nodal recurrence varies by clinical staging. For patients who were initially clinically N0, recurrence rates were 2% vs. 22% for patients who were initially clinically N-positive [clinical N1 disease (cN1)], with presence of US detected abnormal lateral neck lymph nodes.
Moreover, the risk of recurrence in pN1 patients varied by the number of positive lymph nodes (4% for less than 5 nodes vs. 19% for more than 5 nodes). Furthermore, extra-nodal extension was associated with 24% risk of recurrence and possibly a worse disease-specific survival. This study by Randolph et al. (19) has paved the way for the 2015 ATA guidelines to define the criteria for upscaling from low to intermediate risk as pN1 with more than 5 involved lymph nodes (0.2–3 cm). pN1 with extra-nodal extension and more than 3 involved lymph node is considered to harbour about 40% risk for structural disease recurrence.
In their retrospective analysis, Kluijfhout et al. (20) sought to determine how often a completion thyroidectomy would be recommended based on the 2015 ATA guidelines if lobectomy was initially performed in patients with 1–4 cm WDTC without preoperatively known risk factors. They reviewed 1,000 patients operated for WDTC and found that 287 (28.7%) would have been eligible for lobectomy as the initial operation according to the recent NCCN and ATA guidelines. In their study, nearly half of the patients with 1–4 cm WDTC who were eligible for lobectomy according to current NCCN and ATA guidelines (122/287, 42.5%) required completion thyroidectomy based on the final postoperative histopathological characteristics. In their study (20), incidental positive lymph nodes were found in 17.1% of patients eligible for HT (49/287). Of note, we report only 2 out of 46 (4.3%) patients that were upscaled due to the nodal status (patients with macroscopic lymph node involvement were of course not included). The discrepancy may be explained by the fact that our clinical preoperative evaluation is done meticulously by experienced ultrasonographists, cytologists, endocrinologists and head and neck surgeons together in a multidisciplinary dedicated team. Moreover, at the end of their discussion, Kluijfhout et al. (20) mention the limitations of their study. One of the limitations mentioned, is that they used any positive lymph nodes within the specimen as high-risk characteristic, whereas patients with ≤5 lymph nodes (smaller than 0.2 cm) are still considered ATA low risk. Since they didn’t perform prophylactic neck dissections and all patients with clinically N1 were excluded, none of their patients had more than 5 lymph nodes removed. It is possible that of the patients without available size of the lymph node metastasis, some contained micrometastatic (smaller than 0.2 cm) disease and would have been considered ATA low risk. Thus, they state, that the rates of completion TT in their study may be overestimated.
In our study, none of the patients included had undergone a prophylactic central lymph node dissection. Since we have excluded patients who underwent neck dissection, all lymph nodes detected in the 301 patients’ cohort, were found incidentally on histopathologic examination. Six of those 301 patients (2.0%) had incidental lymph nodes detected in their final histopathology specimens. None of the patients had more than 5 positive lymph nodes removed. Of these, only in 1 patient (0.3%), a single positive microscopic lymph was detected. This finding didn’t alter the patient’s risk stratification. Moreover, none of these six patients with incidental lymph nodes detected in their final histopathology specimens was upscaled regarding risk stratification, thus none of them was included in the 46 upscaled patients’ cohort.
Lang et al. (21) showed overall similar upscaling rates [of the 600 patients eligible for lobectomy, 257 (42.8%) had ≥1 unrecognized histological high-risk feature before surgery] and, interestingly, they included positive microscopic margins and multifocality as high-risk factors. When evaluating the high-risk factors that were not identified before surgery, they noted that only lymphovascular invasion was an independent factor for incomplete response following TT and RAI. Murthy et al. (22) performed a similar study in which they demonstrated 59.1% of the patients with unifocal tumors measuring 1–4 cm who were preoperatively eligible for lobectomy needed a completion thyroidectomy based on the adverse features in the histopathology report. Only one study demonstrated a slightly higher 10-year relative OS for TT as opposed to thyroid lobectomy (98.4% vs. 97.1%, respectively, P<0.05) and a slightly lower 10-year recurrence rate (7.7% vs. 9.8%, respectively, P<0.05) (23). Adam et al.’s updated analysis of an earlier study demonstrated that the OS advantage seen for patients with 1–4 cm PTC who underwent TT disappeared when further adjustment was made for additional variables related to complexity and severity of illness (24). This lack of OS advantage was also seen when the group was subdivided into patients with 1–2 and 2–4 cm PTC (25). Two additional studies that analyzed the SEER database failed to demonstrate any significant difference in survival when comparing TT with lobectomy (2,26).
WDTC may be multifocal and often involves both lobes (27). About 30% of the low-risk patients with WDTC harbor contralateral cancers (28) with yet indeterminate clinical significance. PTC is often multifocal, not rarely involving both lobes. There are several studies demonstrating a lower risk of locoregional disease recurrence following TT as opposed to lobectomy (5). However, with meticulous proper patient selection, less than 1–4% locoregional recurrence rates and less than 10% completion thyroidectomy rates can be achieved following thyroid lobectomy (4,5). Moreover, the few recurrences that develop during long-term follow-up can be easily detected and treated appropriately with no impact on survival (4,5).
Nowadays, most authors don’t consider multifocal disease as an indication for TT, since most studies support the findings that multifocality is not an independent risk factor for recurrence of PTC, demonstrating that lobectomy is effective for most patients with unilateral multifocal PTC (5,29-32).
Our study has several limitations that bear mention. First, we acknowledge the inherent potential for selection bias in reviewing patients from a high-volume tertiary academic center. Second, the majority of our cohort was operated prior to 2015, therefore we only had limited data regarding the effect of the recent 2015 ATA guidelines on patients’ risk assessment and upscaling assessment. The majority of our patients were operated in light of the ATA 2009 guidelines (33) thus a higher rate of TT was performed as compared to the rate that could have been performed in light of the ATA 2015 guidelines. The latter may lead to an internal bias due to the fact that the whole specimen was available for pathological evaluation. Moreover, the fact that the majority of our cohort was operated according to the ATA 2009 guidelines limited our ability to define the clinical implications of our findings regarding the upscaling rate. Thus, the data regarding follow-up of our cohort’s patients is out of the scope of this current study.
Only 14 patients were operated after the 2015 ATA guidelines, of which only 2 were upscaled. No significant predictors of upscaling were detected apart from tumor size. We noted that among patients operated prior to the application of the 2015 ATA guidelines, larger tumors were more common in the upscaled group (1.6±0.7 vs. 1.9±0.8 cm, P=0.039).
Third, we did not take the BRAF V600E status into account since we did not routinely perform mutational analyses. Lastly, there may not be complete agreement between the size of the malignant lesion on the pathology exam and the size on US. The size of the tumors included in this study was extracted from the final histopathology reports since it was available for all patients.
We report a 15% rate of postoperative upscaling of the level of risk which necessitated completion thyroidectomy due to findings of high-risk features in patients who had been preoperatively stratified as low-risk WDTC. Our findings in a large tertiary center present lower rates of upscaling than that mentioned in other reports. These findings may be explained due to the meticulous preoperative evaluation that is done in our institution by highly experienced ultrasonographists, cytologists, endocrinologists and head and neck surgeons together in a multidisciplinary dedicated team. This emphasizes the great importance preoperative evaluation prior to tailoring a treatment plan individualized to each patient, especially considering HT vs. TT. Meticulous preoperative evaluation diminishes the cases that intraoperatively the surgeon encounters surprising findings that mandate extreme change of the preoperative plan.
Conclusions
In our opinion, in the view of the current trends towards more conservative management of patients with WDTC, and the fine balance between surgical complication risks in more extensive surgery and preserving an adequate minimal risk of recurrence and metastasis—the possible risk for the need for completion thyroidectomy after an initial lobectomy for patients who were preoperatively stratified as low risk for disease-specific recurrence and were upscaled postoperatively to high risk according to our current analysis (15%), is acceptable. We propose unilateral lobectomy as a viable alternative to TT for selected patients with WDTC between 1–4 cm in size, that should be further considered with the patient. Meticulous preoperative personalized evaluation by an experienced multidisciplinary dedicated team is crucial.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-105
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-105
Peer Review File: Available at https://dx.doi.org/10.21037/gs-21-105
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-105). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of the Tel-Aviv Sourasky Medical Center (TLV-704-16) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zerey M, Prabhu AS, Newcomb WL, et al. Short-term outcomes after unilateral versus complete thyroidectomy for malignancy: a national perspective. Am Surg 2009;75:20-4. [Crossref] [PubMed]
- Mendelsohn AH, Elashoff DA, Abemayor E, et al. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg 2010;136:1055-61. [Crossref] [PubMed]
- Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg 1992;164:658-61. [Crossref] [PubMed]
- Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012;151:571-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156-65. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J Clin Endocrinol Metab 2019; Epub ahead of print. [Crossref] [PubMed]
- Sultana S, Nahar N, Begum F, et al. Management of patients with differentiated thyroid carcinoma-SNMB guidelines. Bangladesh Journal of Nuclear Medicine 2015;18:73-84. [Crossref]
- Sharifi A, Shojaeifard A, Soroush A, et al. Predictors of regional lymph node recurrence after initial thyroidectomy in patients with thyroid cancer. J Thyroid Res 2016;2016:4127278 [Crossref] [PubMed]
- Sezer A, Celik M, Yilmaz Bulbul B, et al. Relationship between lymphovascular invasion and clinicopathological features of papillary thyroid carcinoma. Bosn J Basic Med Sci 2017;17:144-51. [Crossref] [PubMed]
- Kandil E, Krishnan B, Noureldine SI, et al. Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec 2013;75:6-17. [Crossref] [PubMed]
- Stoll SJ, Pitt SC, Liu J, et al. Thyroid hormone replacement after thyroid lobectomy. Surgery 2009;146:554-8; discussion 558-60. [Crossref] [PubMed]
- Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958-64; discussion 964-6. [Crossref] [PubMed]
- Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447-63. [Crossref] [PubMed]
- Grant CS, Hay ID, Gough IR, et al. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery 1988;104:954-62. [PubMed]
- Chan S, Karamali K, Kolodziejczyk A, et al. Systematic review of recurrence rate after hemithyroidectomy for low-risk well-differentiated thyroid cancer. Eur Thyroid J 2020;9:73-84. [Crossref] [PubMed]
- Rajjoub SR, Yan H, Calcatera NA, et al. Thyroid lobectomy is not sufficient for T2 papillary thyroid cancers. Surgery 2018;163:1134-43. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Kluijfhout WP, Pasternak JD, Lim J, et al. Frequency of high-risk characteristics requiring total thyroidectomy for 1-4 cm well-differentiated thyroid cancer. Thyroid 2016;26:820-4. [Crossref] [PubMed]
- Lang BH, Shek TW, Wan KY. The significance of unrecognized histological high-risk features on response to therapy in papillary thyroid carcinoma measuring 1-4 cm: implications for completion thyroidectomy following lobectomy. Clin Endocrinol (Oxf) 2017;86:236-42. [Crossref] [PubMed]
- Murthy SP, Balasubramanian D, Subramaniam N, et al. Prevalence of adverse pathological features in 1 to 4 cm low-risk differentiated thyroid carcinoma. Head Neck 2018;40:1214-8. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375-81; discussion 381-4. [Crossref] [PubMed]
- Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg 2014;260:601-5; discussion 605-7. [Crossref] [PubMed]
- Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol 2005;12:81-9. [Crossref] [PubMed]
- Barney BM, Hitchcock YJ, Sharma P, et al. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck 2011;33:645-9. [Crossref] [PubMed]
- Connor MP, Wells D, Schmalbach CE. Variables predictive of bilateral occult papillary microcarcinoma following total thyroidectomy. Otolaryngol Head Neck Surg 2011;144:210-5. [Crossref] [PubMed]
- Koo BS, Choi EC, Park YH, et al. Occult contralateral central lymph node metastases in papillary thyroid carcinoma with unilateral lymph node metastasis in the lateral neck. J Am Coll Surg 2010;210:895-900. [Crossref] [PubMed]
- Haugen BR. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer 2017;123:372-81. [Crossref] [PubMed]
- Geron Y, Benbassat C, Shteinshneider M, et al. Long-term outcome after hemithyroidectomy for papillary thyroid cancer: a comparative study and review of the literature. Cancers (Basel) 2018;11:26. [Crossref] [PubMed]
- Harries V, Wang LY, McGill M, et al. Should multifocality be an indication for completion thyroidectomy in papillary thyroid carcinoma? Surgery 2020;167:10-7. [Crossref] [PubMed]
- Huang H, Liu S, Xu Z, et al. Long-term outcome of thyroid lobectomy for unilateral multifocal papillary carcinoma. Medicine (Baltimore) 2017;96:e7461 [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]


