
Clinical anatomy of the inferior labial gland: a narrative review
Introduction
The salivary glands in the oral cavity can be divided into two main categories: major and minor. The major salivary glands comprise the parotid, submandibular, and sublingual glands. Collectively, they are responsible for 95% to 97% of saliva production. The minor salivary glands produce the remainder. They are mainly concentrated in the buccal mucosa, labial mucosa, lingual mucosa, gingival mucosa (especially in the retromolar region), soft/hard palate, and floor of the mouth (1,2). Approximately 800–1,000 minor salivary glands are located deep to the oral mucosa, and saliva from their tissues drains directly into the oral cavity without passing through a duct. The minor salivary glands are poorly visualized on computed tomography (CT) and/or magnetic resonance imaging (MRI), but in some cases can be sources of diseases such as tumors, cysts, inflammation and infections. They are also used as material for diagnosing autoimmune diseases that cause xerostomia. The labial gland is one of the minor salivary glands. It lies in the adipose tissue developed in the labial mucosa. It contributes to providing appropriate moisture to the lips. Regardless of its importance in clinical practice, there are no medical literature that comprehensively reviewed the inferior labial gland. In this article, we present a literature review of the inferior labial gland from a clinical and anatomical perspective. We also present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/gs-21-143).
Methods
A database search using PubMed and Google Scholar was conducted on January 13, 2021 without any language limitations. The following keywords were used in the search: “lower labial salivary gland”, “lower labial gland”, “inferior labial salivary gland”, AND “inferior labial gland”.
Results
A total of 48 articles were found in the database. One abstract, two books, and two articles written in Chinese language were excluded. As a result, 43 articles were included and analyzed.
Discussion
Anatomy
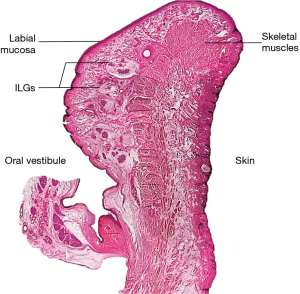
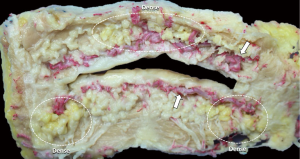
The labial gland is one of the minor salivary glands, which are mainly found beneath the mucosal epithelium of the upper and lower lip and within the orbicularis oris muscle fibers (3) (Figure 1). The upper labial glands are on average distributed 11 mm outward from the corner of the mouth, while the inferior labial glands are on average widely distributed 16 mm outward from the corner of the mouth. The upper labial glands are densely located between and scattered outside the corners of the mouth; the inferior labial glands are scattered between and densely located outside the corners of the mouth (4). In other words, there are many well-developed labial glands in the upper lip, but only a few small labial glands are distributed in the lower lip (4) (Figure 2). A typical labial gland weighs around 50 mg and a density of 5–8 glands per square centimeter on the inner surface of the lips is usual. Stimulated flow from the labial salivary gland is around 5 mL/min/cm2 (5). The inferior labial glands mainly consist of one duct that houses serous and mucous acini cells but produces mostly mucous secretions. Held in place by connective tissue, the inferior labial glands along with the other minor salivary glands are not encapsulated in such tissue but rather surrounded by it (1).
Innervation
The major salivary glands are dominated by both parasympathetic and sympathetic nerves while the minor salivary glands are not innervated autonomically (6). Rather, the inferior labial glands are innervated by the inferior labial branch of the mental nerve (7,8) (Figure 3). The inferior labial branches go to the inferior labial region and branch off the autonomic fibers to the labial glands (8). The postganglionic fibers in the sympathetic division usually originate from the external carotid plexus via the maxillary and inferior alveolar arteries. As the mental nerve communicates with the marginal mandibular branch of the facial nerve, the external carotid plexus via the facial artery is also a candidate of the pathway of the postganglionic fibers.
The postganglionic fibers of the parasympathetic division via the mental nerve have not been much discussed. Segade et al. (1987) found that the otic ganglion in pigs innervates not only the parotid gland but also all other branches of the mandibular nerve such as the inferior alveolar and lingual nerves (9). Thus, the postganglionic fibers could originate from the otic ganglion via the mental nerve.
Blood supply
Branching from the facial artery, the inferior labial artery is the main blood supplier to the lower lip (see Figure 1). There are variations in which the inferior labial artery also acts as a supplementary or main supplier to the lower lip, but they are rare (10). The inferior labial artery runs along the level of the vermillion borders of the lower lip, mostly submucosally. This position is determined to be between the oral mucosa and the orbicularis oris muscle. From there, the inferior labial artery branches off, providing an accessory blood supply to the structures located in the mucosa including the inferior labial glands (11).
Function
The inferior labial gland, like all the minor salivary glands, has no specific neuronal regulation. These glands are mainly under muscarinic control and primarily use the Na+-K+-2Cl− cotransporter to drive transepithelial anion transport and also fluid secretion (12). Thereby, they continuously secrete small amounts of saliva to provide constant lubrication of the oral surface. In total, the minor salivary glands together produce around 10% of the entire salivary volume (13). In view of the large surface area they encompass in comparison to the major glands, each of which has a single large duct, their role in sustained and efficient saliva production for the oral cavity is even more important, especially when pathology affecting the innervation of the major salivary glands makes their role in creating a moist oral mucosa essential.
Salivary antibody production is another major function of the inferior labial gland. Antibodies of each immunoglobin isotype to the antigens of several oral streptococcal bacteria have been detected in saliva collected from the labial salivary glands. This immune defense significantly hampers the growth of mutans streptococci after dental prophylaxis (14). Therefore, the ability of these glands to secrete mainly IgA (15,16) along with IgG (16) and IgM (17) is vital in the immune defense against pathogens mounted by the oral cavity.
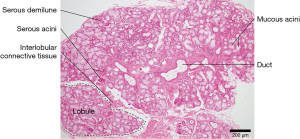
The mucinous acini cells found in the inferior labial glands produce other salivary proteins besides immunoglobins that influence the oral microbiome and the overall health of the oral cavity (Figure 4). Mucins are highly glycosylated proteins mainly produced by mucous acinar cells such as those in the inferior labial glands (18). Owing to their hydrophilic nature, mucins can serve as a protective barrier for the structures in the mouth against bacterial colonization or bacterial proteases. MUC5B is a particular type of mucin secreted by the labial salivary gland that allows S. sanguinis to coexist with S. mutans, thus hampering the formation of a single S. mutans colony and its subsequent biofilm (19,20). MUC1 is another mucin often produced by the labial salivary glands that is involved in signal transduction and scaffolding to hold the salivary proteins in place by forming a mucus layer. It therefore provides a framework for MUC5B to be held in place, enabling its influence on the oral microbiome to take effect (21,22).
Lysozymes are also produced by the labial salivary glands. They hydrolyze the B-1,4-glycosidic bonds between N-acetylmuramic acid and N-acetyl-D-glucosamine in the polysaccharide layer of the Gram-positive bacterial cell wall, so they are bactericidal. Aside from their ability to lyse harmful bacteria, lysozymes serve to aggregate oral bacteria, which facilitates their clearance from the oral cavity (23).
Aging
Studies have shown that the inferior labial salivary glands undergo age-related changes in form and function. In terms of structure, they show increased ductal and connective tissue volumes and fatty infiltration. There is also a rise in the number of inflammatory cell foci (24-27). Although the inferior labial salivary gland shows degenerative changes with age, recent studies by Eliasson et al. conclude that there is no change in the rate of salivary secretion. As for IgA secretion, the inferior labial glands of older subjects secreted saliva with a higher IgA concentration (28).
Diagnostic value
With the surgical failure and risk of obtaining biopsies from the major salivary glands, excision and biopsy of the inferior labial minor salivary glands have been used as a plausible alternative. Located on the inner surfaces of the lips, the inferior labial glands are easily accessible and readily available for biopsy. Since major exocrine disorders such as Sjogren’s syndrome (SS) and cystic fibrosis are reflected in the labial glands, the inferior labial glands are important diagnostic materials for them (29). The following text includes an in-depth review of current research surrounding the use of inferior labial glands for elucidating and potentially diagnosing major systemic diseases.
Primary Sjogren’s syndrome & non-Hodgkin’s lymphoma
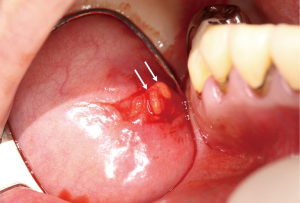
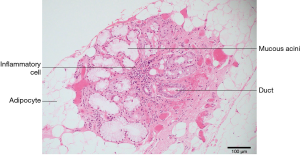
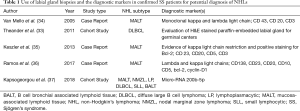
Primary Sjogren’s syndrome is a chronic autoimmune disease affecting the exocrine glands and other organs. It is characterized by lymphocytic infiltration of the salivary and lacrimal glands leading to progressive loss of the glandular parenchyma with a decrease of function, leading in turn to chronic salivary hypofunction and keratoconjunctivitis sicca (30). Labial salivary gland biopsy has long been considered the gold standard for diagnosing patients with primary Sjogren’s syndrome (Figures 5,6). It is often used when other diagnostic measures and laboratory values are inconclusive (31). Focal lymphocytic sialadenitis found in the inferior labial glands is characteristic of Sjogren’s syndrome. In the biopsy procedure, around six to seven glands with notable swelling are harvested and the inflammatory infiltrates are scored and graded according to the method of Greenspan and Daniels (32). Development of non-Hodgkin’s lymphoma (NHL) is the major adverse outcome of Sjogren’s syndrome (33), and recent studies have established the predictability and diagnostic capacity of inferior labial gland biopsies and their value in early diagnosis of NHLs developed from SS (33-37). Table 1 (33-37) summarizes the use of labial gland biopsies and the diagnostic markers used in confirmed SS patients for potential diagnosis of NHLs over the past 20 years. Increasing numbers of case reports and cohort studies exploring the use of inferior labial salivary glands to diagnose NHLs in patients with SS strongly suggest that further establishment and exploration of a standardized testing mechanism should be incorporated into the SS biopsy process to capture the possibility of NHL development comprehensively.
Full table
Cystic fibrosis (CF)
Currently, CF is diagnosed through clear clinical presentations and symptoms, the sweat chloride test, and genetic testing for the two known disease-causing CFTR mutations. However, for patients with limited phenotypes, the diagnostic process can be more complex. Other methods such as nasal potential difference measurements or current intestinal measurements can be used (38). Labial salivary gland biopsy is another potential diagnostic material that can be explored and used for patients who fall into this pathology. Although the inferior labial glands of patients with and without CF have the same ultrastructure, those from CF patients contain more mucin (39). Aside from mucin accumulation, it has also been shown by Sweney et al. that most pediatric patients display acinar plugs of eosinophilic material in the inferior labial glands upon biopsy, which normally appear as one ages (40). Also, when the biopsy is processed for fixation, the epithelium of the mucous membrane separates from the glandular tissue; in normal biopsies the connective tissue layers remain intact (39). Most recently, using the non-invasive technique of optical coherence tomography, a lower density of labial salivary glands has been demonstrated in CF patients than in a group without the disease (41).
Neonatal hemochromatosis (NH)
NH is a fatal neonatal iron storage disease in which iron is deposited at hepatic and extrahepatic sites, leading to liver and multiorgan failure (42). Because the onset of the disease occurs in utero, affected infants are often either stillborn, premature, or small with signs of organ failure at birth (43). Therefore, early diagnosis is important for extreme medical intervention because the condition worsens over time (44). Recent case studies have revealed iron deposits in the inferior labial salivary glands of NH patients. However, the sample is small so false positives or negatives are likely (45). Nonetheless there are proponents for the use of inferior labial salivary gland biopsy along with clear clinical presentations for diagnosing NH, and more researches are indicated to incorporate inferior labial gland biopsy formally into the diagnostic protocol of NH (46,47).
Ocular sarcoidosis
Sarcoidosis is an immune-mediated inflammatory disorder that can present as uveitis. Some features suggesting sarcoidosis uveitis include the following five signs: granulomatous iritis with fat keratic precipitates, iris nodules, string of vitreous opacities, retinal perivasculitis, and spotty retinochoroidal exudates. Because these features are common and because ocular presentations of sarcoidosis potentially precede systemic signs, sarcoidosis must be considered as an underlying cause of undiagnosed uveitis (48-51). With a low diagnostic yield for sarcoidosis, inferior labial gland biopsy has been shown to be useful for diagnosing sarcoid uveitis in patients with confirmed sarcoidosis. It has been suggested that inferior labial gland biopsy is useful as a second-line investigation for patients with granulomatous uveitis and radiological patterns compatible with sarcoidosis for an earlier intervention to treat the uveitis (52).
Other pathologies
Mucocele
Oral mucoceles that typically involve the minor salivary glands found in the lower lip and the buccal mucosa originate from a traumatic incident leading to duct laceration and leakage (53). Focusing on the inferior labial glands, a lacerated duct will lead to a spread of mucin around the inferior labial submucosal connective tissue. After mucus extravasation, an immune response follows that leads to tissue granulation and inflammatory cell inundation in order to control the mucin spread. Over time the area is pseudoencapsulated with fibrous tissue (54,55). Like any mucocele that originates from mucus extravasation, inferior labial gland mucoceles can undergo sequences of appearance and disappearance (56). Conventional treatment modalities for inferior labial glands include simple surgical excision using the underlying orbicularis oris muscle as a cleavage plane (57). Other nonsurgical treatments include intralesional corticosteroid therapy, cryosurgery, micromarsupialization, and carbon dioxide laser (58).
Tumors
The minor salivary glands are the second most common locations for salivary gland tumors following the parotid gland (59,60). The palate is the most common location for minor salivary gland tumors, and the lips are the second most common. Tumors arise more commonly in the superior than the inferior labial salivary glands. However, a tumor arising from the inferior labial salivary glands is more likely to be malignant. The most common malignancy arising from the inferior labial glands is mucoepidermoid carcinoma (61,62).
Conclusions
The human labial glands, a subset of the minor salivary glands, are important structures for oral health. Their continual secretion of saliva is important for immune defense by preventing dryness of the oral tissue around the lips and by producing antibacterial secretions.
Tumoral lesions that occur in the lower lip include cystic diseases such as mucocele and dermoid cysts, benign tumors such as fibroma and schwannoma, and malignant tumors such as mucoepidermoid carcinoma and adenocarcinoma. Among these, about half or more of the tumoral lesions that occur in the lower lip are reported to be malignant tumors derived from the inferior labial glands (59,63). We must be very careful in differentiating these diseases.
The inferior labial gland is expected to be used not only for its original salivary gland function and as diagnostic material for autoimmune diseases, but also as transplant material for improving severe dry eye symptoms (64,65). However, our knowledge of the minor salivary glands, including the labial glands, is still limited compared to the major salivary glands. We hope that further research focused on the function of the labial glands and the derivation of new diagnoses and techniques will help to improve our understanding of their role in health and disease.
Acknowledgments
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge, which can then improve patient care. Therefore, these donors and their families deserve our highest gratitude (Iwanaga et al. Clin Anat 2021;34:2-4).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-143
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-143). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kessler AT, Bhatt AA. Review of the Major and Minor Salivary Glands, Part 1: Anatomy, Infectious, and Inflammatory Processes. J Clin Imaging Sci 2018;8:47. [Crossref] [PubMed]
- Iwanaga J, Nakamura K, Alonso F, et al. Anatomical study of the so-called "retromolar gland": Distinguishing normal anatomy from oral cavity pathology. Clin Anat 2018;31:462-5. [Crossref] [PubMed]
- Ellis GL, Auclair PL. Tumors of the Salivary Glands. AFIP Atlas of Tumor Pathology. 4th series. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2008.
- Kamijo Y. Oral Anatomy. Tokyo: Anatom Publisher. 1969
- Speirs RL. Secretion of saliva by human lip mucous glands and parotid glands in response to gustatory stimuli and chewing. Arch Oral Biol 1984;29:945-8. [Crossref] [PubMed]
- du Toit DF, Nortjé C. Salivary glands: applied anatomy and clinical correlates. SADJ 2004;59:65-6, 69-71, 73-4. [PubMed]
- Alantar A, Roche Y, Maman L, et al. The lower labial branches of the mental nerve: anatomic variations and surgical relevance. J Oral Maxillofac Surg 2000;58:415-8. [Crossref] [PubMed]
- Iwanaga J, Saga T, Tabira Y, et al. The clinical anatomy of accessory mental nerves and foramina. Clin Anat 2015;28:848-56. [Crossref] [PubMed]
- Segade LA, Suarez Quintanilla D, Suarez Nuñez JM. The postganglionic parasympathetic fibers originating in the otic ganglion are distributed in several branches of the trigeminal mandibular nerve: an HRP study in the guinea pig. Brain Res 1987;411:386-90. [Crossref] [PubMed]
- Edizer M, Mağden O, Tayfur V, et al. Arterial anatomy of the lower lip: a cadaveric study. Plast Reconstr Surg 2003;111:2176-81. [Crossref] [PubMed]
- Cotofana S, Pretterklieber B, Lucius R, et al. Distribution Pattern of the Superior and Inferior Labial Arteries: Impact for Safe Upper and Lower Lip Augmentation Procedures. Plast Reconstr Surg 2017;139:1075-82. [Crossref] [PubMed]
- Turner RJ, Paulais M, Valdez II, et al. Ion transport and signalling in human labial glands. Arch Oral Biol 1999;44:S15-9. [Crossref] [PubMed]
- Dawes C, Wood CM. The composition of human lip mucous gland secretions. Arch Oral Biol 1973;18:343-50. [Crossref] [PubMed]
- Smith DJ, Taubman MA. Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol 1990;10:273-81. [Crossref] [PubMed]
- Crawford JM, Taubman MA, Smith DJ. Minor salivary glands as a major source of secretory immunoglobin A in the human oral cavity. Science 1975;190:1206-9. [Crossref] [PubMed]
- Smith DJ, Taubman MA, King WF. Immunological features of minor salivary gland saliva. J Clin Immunol 1987;7:449-55. [Crossref] [PubMed]
- Smith DJ, Taubman MA, Ali-Salaam P. Immunoglobulin isotypes in human minor gland saliva. J Dent Res 1991;70:167-70. [Crossref] [PubMed]
- Amerongen AV, Bolscher JG, Veerman EC. Salivary mucins: protective functions in relation to their diversity. Glycobiology 1995;5:733-40. [Crossref] [PubMed]
- Frenkel ES, Ribbeck K. Salivary mucins promote the coexistence of competing oral bacterial species. ISME J 2017;11:1286-90. [Crossref] [PubMed]
- Frenkel ES, Ribbeck K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl Environ Microbiol 2015;81:332-8. [Crossref] [PubMed]
- Gibbins HL, Proctor GB, Yakubov GE, et al. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis 2014;20:707-13. [Crossref] [PubMed]
- Gabryel-Porowska H, Gornowicz A, Bielawska A, et al. Mucin levels in saliva of adolescents with dental caries. Med Sci Monit 2014;20:72-7. [Crossref] [PubMed]
- Lynge Pedersen AM, Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 2019;80:S3-S12. [Crossref] [PubMed]
- Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale 1980;8:187-200. [PubMed]
- Drummond JR, Chisholm DM. A qualitative and quantitative study of the ageing human labial salivary glands. Arch Oral Biol 1984;29:151-5. [Crossref] [PubMed]
- Syrjänen S. Age-related changes in structure of labial minor salivary glands. Age Ageing 1984;13:159-65. [Crossref] [PubMed]
- Katona K, Elekes E, Farkas N, et al. Image analysis of fatty infiltration in labial salivary gland biopsies: extent and its correlation to age, obesity and diabetes. J Oral Pathol Med 2017;46:537-42. [Crossref] [PubMed]
- Eliasson L, Birkhed D, Osterberg T, et al. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur J Oral Sci 2006;114:494-9. [Crossref] [PubMed]
- Izutsu KT, Schubert MM, Truelove EL, et al. Use of human minor salivary glands in basic and applied secretion research. J Dent Res 1987;66:654-9. [Crossref] [PubMed]
- Daniels TE, Fox PC. Salivary and oral components of Sjögren's syndrome. Rheum Dis Clin North Am 1992;18:571-89. [Crossref] [PubMed]
- St Clair EW, Lackey VD. Chapter 73 - Sjögren's Syndrome. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley and Firestein's Textbook of Rheumatology 10th ed. Philadelphia: Elsevier; 2017;1221-44.
- Malladi AS, Sack KE, Shiboski SC, et al. Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjögren's syndrome registry. Arthritis Care Res (Hoboken) 2012;64:911-8. [Crossref] [PubMed]
- Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis 2011;70:1363-8. [Crossref] [PubMed]
- Van Mello NM, Pillemer SR, Tak PP, et al. B cell MALT lymphoma diagnosed by labial minor salivary gland biopsy in patients screened for Sjögren's syndrome. Ann Rheum Dis 2005;64:471-3. [Crossref] [PubMed]
- Keszler A, Adler LI, Gandolfo MS, et al. MALT lymphoma in labial salivary gland biopsy from Sjögren syndrome: importance of follow-up in early detection. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:e28-33. [Crossref] [PubMed]
- Ramos LM, Ferrar TC, Shcaira V, et al. Pp - Malt Lymphoma In Labial Salivary Gland: A Rare Manifestation Of Sjogren’s Syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123:E60 [Crossref]
- Kapsogeorgou EK, Papageorgiou A, Protogerou AD, et al. Low miR200b-5p levels in minor salivary glands: a novel molecular marker predicting lymphoma development in patients with Sjögren's syndrome. Ann Rheum Dis 2018;77:1200-7. [Crossref] [PubMed]
- De Boeck K, Vermeulen F, Dupont L. The diagnosis of cystic fibrosis. Presse Med 2017;46:e97-e108. [Crossref] [PubMed]
- Doggett RG, Bentinck B, Harrison GM. Structure and ultrastructure of the labial salivary glands in patients with cystic fibrosis. J Clin Pathol 1971;24:270-82. [Crossref] [PubMed]
- Sweney LR, Hedrick MC, Meskin LH, et al. The involvement of the labial mucous salivary gland in patients with cystic fibrosis. II. The heterozygote state. Pediatrics 1967;40:421-4. [PubMed]
- Nowak JK, Grulkowski I, Karnowski K, et al. Optical coherence tomography identifies lower labial salivary gland surface density in cystic fibrosis. PLoS One 2015;10:e0117517 [Crossref] [PubMed]
- Knisely AS. Neonatal hemochromatosis. Adv Pediatr 1992;39:383-403. [PubMed]
- Whitington PF. Fetal and infantile hemochromatosis. Hepatology 2006;43:654-60. [Crossref] [PubMed]
- Grabhorn E, Richter A, Burdelski M, et al. Neonatal hemochromatosis: long-term experience with favorable outcome. Pediatrics 2006;118:2060-5. [Crossref] [PubMed]
- Chan KC, Edelman M, Fantasia JE. Labial salivary gland involvement in neonatal hemochromatosis: a report of 2 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:e27-30. [Crossref] [PubMed]
- Knisely AS, O'Shea PA, Stocks JF, et al. Oropharyngeal and upper respiratory tract mucosal-gland siderosis in neonatal hemochromatosis: an approach to biopsy diagnosis. J Pediatr 1988;113:871-4. [Crossref] [PubMed]
- Murray KF, Kowdley KV. Neonatal hemochromatosis. Pediatrics 2001;108:960-4. [Crossref] [PubMed]
- Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol 1996;114:593-9. [Crossref] [PubMed]
- Rizzato G, Angi M, Fraioli P, et al. Uveitis as a presenting feature of chronic sarcoidosis. Eur Respir J 1996;9:1201-5. [Crossref] [PubMed]
- Rothova A, Buitenhuis HJ, Meenken C, et al. Uveitis and systemic disease. Br J Ophthalmol 1992;76:137-41. [Crossref] [PubMed]
- Rothova A, Alberts C, Glasius E, et al. Risk factors for ocular sarcoidosis. Doc Ophthalmol 1989;72:287-96. [Crossref] [PubMed]
- Blaise P, Fardeau C, Chapelon C, et al. Minor salivary gland biopsy in diagnosing ocular sarcoidosis. Br J Ophthalmol 2011;95:1731-4. [Crossref] [PubMed]
- More CB, Bhavsar K, Varma S, et al. Oral mucocele: A clinical and histopathological study. J Oral Maxillofac Pathol 2014;18:S72-7. [Crossref] [PubMed]
- Hayashida AM, Zerbinatti DC, Balducci I, et al. Mucus extravasation and retention phenomena: a 24-year study. BMC Oral Health 2010;10:15. [Crossref] [PubMed]
- Chaitanya P, Praveen D, Reddy M. Mucocele on Lower Lip: A Case Series. Indian Dermatol Online J 2017;8:205-7. [Crossref] [PubMed]
- Karthikeyan M, Varghese AK, Vasupradha G, et al. Mucocele: A diagnostic dilemma!! J Pharm Bioallied Sci 2016;8:S168-70. [PubMed]
- Zeng QC, Mandel L. Surgical Management of the Labial Mucocele. N Y State Dent J 2019;85:42-4.
- Ata-Ali J, Carrillo C, Bonet C, et al. Oral mucocele: Review of the literature. J Clin Exp Dent 2010;2:e18-21. [Crossref]
- Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol 1985;146:51-8. [Crossref] [PubMed]
- Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177-84. [Crossref] [PubMed]
- Neville BW, Damm DD, Weir JC, et al. Labial salivary gland tumors. Cancer 1988;61:2113-6. [Crossref] [PubMed]
- Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg 2005;63:805-10. [Crossref] [PubMed]
- Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol 1988;66:323-33. [Crossref] [PubMed]
- Geerling G, Raus P, Murube J. Minor salivary gland transplantation. Dev Ophthalmol 2008;41:243-54. [Crossref] [PubMed]
- Qin Y, Zhang Y, Liang Q, et al. Labial Salivary Gland Transplantation for Severe Dry Eye in a Rhesus Monkey Model. Invest Ophthalmol Vis Sci 2018;59:2478-86. [Crossref] [PubMed]



