
Evidence on reporting guidelines for surgical technique in clinical disciplines: a scoping review protocol
Introduction
Currently, no clear definition of surgical technique is found in the literature or dictionaries. According to the Oxford Dictionary, “surgical” means “used in or connected with surgery” and “technique” stands for “a particular way of doing something, especially in which you have to learn special skills” (1). Therefore, “Surgical Technique” is defined as “The specific way and skills of performing a particular medical operation”. Specifically, the surgical technique that we are concerned with here are the skills of treatments of injuries, diseases or any discomforts or abnormalities in people, by removing abnormalities, repairing affected parts, or implanting substitutions, by cutting open, whether invasive, minimally invasive or non-invasive and whether they are carried out by surgeons or other medical practitioners. In PubMed, 38,208 articles were identified using the search term “surgical technique” [Title/Abstract] (search date Oct 27, 2020) (2). The application of the surgical technique among the first 100 relevant articles is confirmed consistent with this definition. Noteworthy, while the term “operative technique” could be interchangeably used with “surgical technique”, “surgical procedure” is not the same as “surgical technique”. Surgical procedure covers the entire operation, including preoperative, intraoperative, and postoperative process. Surgical technique, on the other hand, specifically focuses more on the intraoperative implementation instead of perioperative care (3,4).
Surgical technique is delicately described as the essential component of the craft and art of surgery (5). A surgical technique can be defined as excellent when it meets several aims, including ensuring the safety of surgery (6), reducing postoperative complications (7), achieving good patient outcomes and making the surgery and future good outcomes reproducible (5). However, the reporting of a surgical technique is of mixed quality, with most at a very minimum level. A systematic review that included 92 surgical case series indicates poor reporting represented by non-use of standard definitions (57%), missing data (66%) and incomplete reporting (70%) etc. (8). In terms of case reports and case series of surgical techniques, few studies have very detailed descriptions of the technique, such as the length of incision, the depth of dissection, appropriate instruments and retractors, and the duration of the procedure (9). Few studies are accompanied by surgical videos. Those with videos are often non-edited lacking corresponding subtitles or audio guidance (10), which is notably different with edited videos. A large number of studies have focused on preoperative preparation, with only a rough description of the steps and a lack of detailed implementation skills or of the “pros” and the “cons” of the reported surgical technique or when this technique is really indicated or when not-indicated (11-13). In terms of randomized controlled trials of surgical techniques, the focus is on the study design and statistics, while the two surgical techniques being compared are not described in details, frequently with no more than two paragraphs (14). The incompleteness, lack of details, and low quality of surgical technique reporting severely limit the evaluation, dissemination, and reproducibility of the surgical technique.
Reporting guidelines are the preferred tool for improving the completeness, detail, transparency, and quality of surgical technique reporting. According to the Equator Network, a reporting guideline is “a checklist, flow diagram, or structured text to guide authors in reporting a specific type of research, developed using explicit methodology. A reporting guideline provides a minimum list of information needed to ensure a manuscript which can be understood by a reader, replicated by a researcher, used by a doctor to make a clinical decision, and included in a systematic review” (15). Although a reporting guideline is not a tool for assessing the methodological quality of an article (16), it can be helpful to authors, reviewers, and journal editors in improving the reporting and educational quality. A survey that included 1,391 authors and 259 reviewers (17) recognized that the earlier an author uses a reporting guideline the more valuable the study is perceived to be, with 77% of reviewers using the reporting guideline in the peer-review process and 60% indicating that the reporting guideline influenced their comments. Owing to the improved recognition of the value of reporting guidelines, a large number of guidelines were published after the publication of CONSORT in 1996 (18). As of October 27, 2020, Equator Network has indexed 442 reporting guidelines (19), with an average of more than 18 newly published or updated reporting guidelines per year in the latest two decades.
However, the 43 reporting guidelines in surgery from pre-search (search date October 17, 2020) in Equator Network have mixed information either describing the discipline covered, the focus on the surgical technique, or the methodology of development. Among the pre-search result, only IDEAL (20) is both focused on surgical technique and covers all clinical disciplines. Nevertheless, the reporting requirement in IDEAL requires the authors to “provide a detailed description” without further specification about the depth or the kind of details are required. A large amount of reporting guidelines is primarily concerned with perioperative management, with insufficient attention, a brief description, or no provision for the surgical technique itself (21-25). Some of the reporting guidelines for certain clinical specialties are very detailed regarding the requirements for surgical technique, including additional details beyond identifying anatomical markers like width, volume, depth, length, distance, angle, and stability of key steps, such as TEVAR (26) for thoracic endovascular aortic repair. Other reporting guidelines addressing a specific surgical technique from other clinical specialties are much less stringent, such as the CORDES (27) for deep endometriosis surgery. Many reporting guidelines in surgery poorly describe the methodology used, e.g., many do not report which databases they have searched (20-23,26,27).
While reporting guidelines for surgical technique exist with incomplete methodological reporting, inconsistent coverage of a surgical discipline, and varying concerns and requirements for surgical technique, a systematic review or scoping review of reporting guidelines for surgical techniques is not found after searching in PubMed and Google Scholar. Due to this gap, there is no way to systematically know the reporting guidelines for surgical techniques with regards to the overall number, the distribution of disciplines, the methodological rigour with which they are developed, the focus on steps, and the extent to which they required detailed description, etc. Most importantly, it is unclear what surgical discipline and technique reporting we need to strengthen and improve the most.
Therefore, given the importance of reporting guidelines for surgical techniques to improve completeness, detail, transparency and quality, the variations in reporting guidelines, and the lack of systematic understanding, we propose to conduct a scoping review for surgical technique reporting guidelines in all clinical disciplines. By the scoping review, we aim to identify the current state of surgical technique reporting guidelines and the areas that need improvement. This protocol is intended to provide the design, steps, details, and considerations for the scoping review.
Protocol design
This scoping review protocol is designed according to the 2020 manual proposed by Joanna Briggs Institute (28). The manual suggests organizing the scoping review protocol in the steps listed below, which our protocol will accordingly include: Step 1: Identifying the research questions; Step 2: Inclusion criteria; Step 3: Search strategy; Step 4: Source of evidence selection; Sepp 5: Data extraction; Step 6: Analysis of the evidence; Step 7: Presentation of the results.
Identifying the research questions
The research questions proposed to answer are mainly in three dimensions: (I) what are the number, distribution of disciplines, and the coverage of pre-/intra-/post-operative procedure of the reporting guidelines? (II) what is the methodology, reporting, assessment, and updating plan of the reporting guidelines? (III) what are the specific requirements and concerns of the items in the reporting guidelines?
The more elaborated questions to be answered are listed below:
- How many reporting guidelines are there regarding surgical technique in different clinical disciplines?
- How many items are in the reporting guidelines?
- What are the demographics of the reporting guidelines in terms of authors, journals, countries and specialties etc.?
- Are there any descriptions about the analysis of the reporting guidelines, including its update plan?
- How many items are about the surgical technique and perioperative care, respectively?
- What are the focuses of the items regarding the surgical technique?
Inclusion criteria
Eligible studies will be included following the criteria below:
- Study topic: surgical technique and surgery on human patients;
- Study concept: reporting checklists/items/guidelines;
- Context: any clinical specialty;
- Publication type: all, including journal articles and grey literature;
- Evidence sources: Equator Network, MEDLINE (via PubMed), Google Scholar and Networked Digital Library of Theses and Dissertations (NDLTD);
- Time frame: no restriction;
- Language: English only;
- Geographic location: all locations.
Search strategy
Equator Network, MEDLINE (via PubMed), Google Scholar and NDLTD are planned to be searched. Equator Network is chosen, considering that it is the largest platform for indexing biomedical reporting guidelines. Since the topic is the biomedical field, MEDLINE (via PubMed) is chosen as one of the premier search platforms in this field. In addition, to avoid any publication bias, Google Scholar and NDLTD are chosen to search for relevant grey literature.
How will we do it
Two teams will conduct independent searches to ensure consistency of search results. When there are inconsistencies, discussions will be held to reach consensus and a final agreed search result. The search will use both keywords and subject headings methods, and will be logically connected with Boolean operators “OR” and “AND”. The search strategy (Table 1) will be adapted to other databases.

Full table
Source of evidence selection
Before the evidence is selected, a consensus and document on the eligibility criteria and elaboration will be achieved. The selection of studies will be carried out by two independent groups in which each person has to receive training before implementation. The training will include detailed eligibility criteria explanation with examples and test, both with selected and excluded cases. Also, a pilot test will be proceeded to refine the selection process. The screening will only get started after 100% agreement is achieved.
Endnote X9 will be used as the screening software. After eliminating duplicates, the screening will be carried out based on title and abstract examination, and then full-text examination. A consensus discussion will be conducted to solve disagreements.
A flowchart of the review process will be drawn in accordance with the PRISMA extension for scoping reviews (PRISMA-ScR) (29), accompanied by an appendix for details of included and excluded sources with reasons.
Data extraction
Corresponding to the research questions to answer, Table 2 summarizes the data to be extracted.
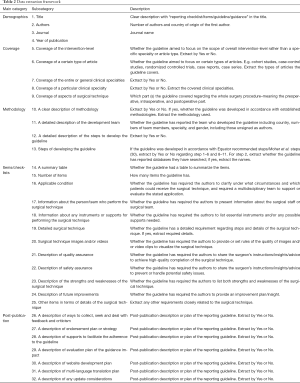
Full table
Analysis of the evidence
Unlike systematic reviews, the scoping review will only summarize and analyze the results without evaluation. Both quantitative data and descriptive qualitative data (Table 2) will be analyzed regarding frequency, distribution, characteristics, and key words etc.
Presentation of the results
The scoping review will summarize the evidence on reporting items for surgical technique in clinical disciplines, identify what is not done well, make practical conclusions, and propose useful insights for improvement.
The scoping review will be written in accordance with the PRISMA-ScR Checklist (29). The results will be presented with both text and visualized figures and tables as appropriate.
Strengths and limitations of the protocol
This protocol’s strengths include its methodological rigour and full consideration of multiple disciplines. The protocol was strictly developed with reference to the classic Joanna Briggs Institute manual, which ensures the rigour of the subsequent scoping review. Additionally, the protocol was discussed by members from multiple disciplines, including methodologists, journal editors and surgeons, making the proposed data to be extracted very detailed and representative. However, the scoping review will only include English reporting guidelines, which induces limited applicability to reporting guidelines in other languages. Moreover, due to the lack of existing tools for evaluating the quality of reporting guidelines, the protocol was only used for summarizing and analyzing, without evaluating the literature. In the future, tools are needed for assessing the quality of reporting guidelines. In this way, a systematic review and relating protocol in this area will be possible.
Conclusions
In summary, this protocol details our plans for an upcoming scoping review. The scoping review will indicate the gaps and efforts needed in surgical technique reporting guidelines.
Acknowledgments
Funding: This project is supported by the AME Reporting Guidelines Research Fund (No. 2020-1016-885) and Lanzhou University Institute of Health Data Science Fund.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-311). AF declares a pending patent (PCT/IT2018/00000S) related to the project. ADLS receives Speaker’s honoraria from Medtronic and is the consultancy of Medela. FD declares a licensed patent (Italian patent application “TO2013A000038”) related to the project. RHP receives Speaker fee from Medtronic and Advisory Board member AstraZeneca. KZ, GSL, XT and SDW are staff of AME publishing company (the publisher of Gland Surgery). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As a scoping review which synthesizes information from published sources, there is no requirement for ethical approval. The scoping review will be published as open access. Meanwhile, academic conferences, mainstream media and other channels will be used for broader dissemination.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Oxford Dictionary - Bethesda, MD: National Library of Medicine, 2020. Available online: https://www.nlm.nih.gov/
- Hayes K, Eid G. Laparoscopic Sleeve Gastrectomy: Surgical Technique and Perioperative Care. Surg Clin North Am 2016;96:763-71. [Crossref] [PubMed]
- Biertho L, Lebel S, Marceau S, et al. Biliopancreatic Diversion with Duodenal Switch: Surgical Technique and Perioperative Care. Surg Clin North Am 2016;96:815-26. [Crossref] [PubMed]
- Hosseinpour AR. The Importance of Surgical Technique. J Surg Tech Proced 2017;1:1001.
- World Health Organization. WHO guidelines for safe surgery: safe surgery saves lives. Available online: https://www.who.int/patientsafety/safesurgery/knowledge_base/SSSL_Brochure_finalJun08.pdf
- Brkic F, Mujic M, Umihanic S, et al. Haemorrhage Rates After Two Commonly Used Tonsillectomy Methods: a Multicenter Study. Med Arch 2017;71:119-21. [Crossref] [PubMed]
- Agha RA, Fowler AJ, Lee SY, et al. Systematic review of the methodological and reporting quality of case series in surgery. Br J Surg 2016;103:1253-8. [Crossref] [PubMed]
- Okhunov Z, Farhan B, Ahmed A, et al. Surgical technique for removal of tined lead for InterStim. Can J Urol 2017;24:8918-20. [PubMed]
- De la Huerta I, Yonekawa Y, Thomas BJ, et al. A Surgical Technique for the Management of Tractional Retinal Detachment in Aggressive Posterior Retinopathy of Prematurity Treated With Intravitreal Bevacizumab. Retina 2019;39:S156-9. [Crossref] [PubMed]
- Stancu B, Grad NO, Mihaileanu VF, et al. Surgical technique of concomitant laparoscopically assisted vaginal hysterectomy and laparoscopic cholecystectomy. Clujul Med 2017;90:348-52. [PubMed]
- Singh SR, Yangzes S, Gupta R, et al. Surgical technique for management of isolated lenticular coloboma with high corneal astigmatism. Indian J Ophthalmol 2018;66:562-4. [Crossref] [PubMed]
- Reddy SSP. Pinhole Surgical Technique for treatment of marginal tissue recession: A case series. J Indian Soc Periodontol 2017;21:507-11. [PubMed]
- Wei S, Guo C, He J, et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients With Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg 2019;154:e190972 [Crossref] [PubMed]
- Equator. What is a reporting guideline. Available online: https://www.equator-network.org/about-us/what-is-a-reporting-guideline/
- Logullo P, MacCarthy A, Kirtley S, et al. Reporting guideline checklists are not quality evaluation forms: they are guidance for writing. Health Sci Rep 2020;3:e165 [Crossref] [PubMed]
- Dewey M, Levine D, Bossuyt PM, et al. Impact and perceived value of journal reporting guidelines among Radiology authors and reviewers. Eur Radiol 2019;29:3986-95. [Crossref] [PubMed]
- Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9. [Crossref] [PubMed]
- Equator. Reporting guidelines. Available online: https://www.equator-network.org/reporting-guidelines/
- Bilbro NA, Hirst A, Paez A, et al. The IDEAL Reporting Guidelines: A Delphi Consensus Statement Stage Specific Recommendations for Reporting the Evaluation of Surgical Innovation. Ann Surg 2021;273:82-5. [Crossref] [PubMed]
- Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156-65. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Farwana R, et al. The PROCESS 2018 statement: Updating Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int J Surg 2018;60:279-82. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Farwana R, et al. The SCARE 2018 statement: Updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2018;60:132-6. [Crossref] [PubMed]
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [Crossref] [PubMed]
- Boutron I, Altman DG, Moher D, et al. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med 2017;167:40-7. [Crossref] [PubMed]
- Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 2010;52:1022-33.e15. [Crossref] [PubMed]
- Vanhie A, Meuleman C, Tomassetti C, et al. Consensus on Recording Deep Endometriosis Surgery: the CORDES statement. Hum Reprod 2016;31:1219-23. [Crossref] [PubMed]
- Aromataris E, Munn Z (editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available online: https://synthesismanual.jbi.global
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169:467-73. [Crossref] [PubMed]
- Moher D, Schulz KF, Simera I, et al. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217 [Crossref] [PubMed]


