
3D-printed, patient-specific DIEP flap templates for preoperative planning in breast reconstruction: a prospective case series
Introduction
The incidence of breast cancer diagnosis has been rising steadily (1) and an increasing number of women are opting for post-mastectomy reconstruction (2). In comparison to implant-based techniques, autologous breast reconstruction based on deep inferior epigastric artery perforator (DIEP) flap yields long-lasting, natural-appearing outcome and is, hence, considered the gold standard reconstruction (3-9).
Due to high individual variation in the vascular anatomy of the DIEA perforators (10), computed tomographic angiography (CTA) is performed to help select ideal perforator and flap design preoperatively (11-16). Advances in the hardware of CTA, such as an increasing number of detector rows (17), and software, such as OsiriX (Pixmeo, Geneva, Switzerland) (18), have ensured its precision, fast speed and reliability.
As a result, the use of CTA in DIEP flap planning has led to reduced postoperative flap complications, such as fat necrosis, flap loss and donor site morbidity (12,19-22). Interestingly, Smit et al. have reported a significant reduction in operating time using CTA in DIEP reconstructions (264 vs. 354 mins, P<0.001) (11). However, Rozen et al. have reported no statistically significant reduction in the total operating time of both unilateral and bilateral cases (P=0.57 and 0.079, respectively) (12). This may be attributed to the fact that current imaging modalities are limited by being displayed on a two-dimensional (2D) surface like a computer screen. Novel ways of utilising the CTA data, such as 3D printing, may enable intuitive spatio-temporal understanding of the involved anatomical structures.
In the last decade, 3D printing has become affordable and readily accessible (23,24). In plastic and reconstructive surgery, 3D printing appears most useful in preoperative planning, designing intraoperative guidance devices, patient education, and building customized implants (25-27). Recently, we have developed an affordable and convenient method of 3D printing for clinicians using free, open-source softwares and desktop 3D printers (28-31). Using this technique, we have 3D-printed patient-specific DIEP templates and utilized them in 20 consecutive patients receiving autologous breast reconstruction with abdominal-wall based free flaps.
This prospective study aims to assess the feasibility and usefulness of 3D designed DIEP perforator templates as a practical tool for DIEP breast reconstruction.
Methods
In this prospective case series of 25 abdominal wall-based flaps in 20 patients over 12-month period (June 2016 to June 2017), we use routine CTA scan data to create patient-specific DIEP templates for preoperative planning. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained through Peninsula Health HREC, approval number PH/3DP, and all participants gave informed consent before taking part.
CTA
CTA was performed using standardized “single-volume” acquisition technique that ensures maximal image quality and minimal radiation exposure, as previously described by Rozen et al. (32). Siemens SOMATOM Sensation 64 multi-detector row computed tomography scanner (Siemens Medical Solutions, Erlangen, Germany) was used and the scan parameters are summarized in Table 1.

Full table
Design of the DIEP template
CTA data is exported from the scanner in DICOM (Digital Imaging and COmmunications in Medicine) format and is processed using free, open-source software (see Figure 1): 3D Slicer (Surgical Planning Laboratory, Boston, MA, USA), Autodesk MeshMixer (Autodesk, Inc., San Rafael, CA, USA), and Cura (Ultimaker, Geldermalsen, Netherlands).

Following this, a 3D image of the patient’s abdominal wall with perforations indicating the location of perforators is created from the DICOM files in 3D Slicer. Using the “threshold” function, a range of Hounsfield unit-derived arbitrary values can be selected to automatically generate a 3D image of the abdominal wall. Scrolling through the axial slices, using “RectangleEffect” tool and erase function, holes and lines are created in the 3D image at the location of DIEA perforators, their intramuscular course and the DIEA pedicle. Similarly, a notch in the 3D image is created at pubic symphysis. The final 3D image is exported in STL (standard tessellation language) format.
In Autodesk MeshMixer, the STL file is made suitable for clinical application. Using “SphereDisc” brush tool, the perforators, their intramuscular course and the DIEA are enlarged to enable a marking pen to fit. Then, the 3D image is cropped around four edges to fit inside a 3D printer. Using “separate” function, anterior surface of the 3D image is isolated and then, using “extrude” function, it is thickened 5 mm into a physical template. Finally, using “smooth” function, the file is smoothed out and then exported in STL format.
In Cura, this final STL file is transformed into a 3D printer-friendly file. 3D slicing software, like Cura, automatically generates support structures and creates an instruction for a 3D printer to follow. This file is exported in G-code format via an external storage device.
3D printing
The templates are 3D-printed in thermoplastic polylactic acid (PLA) filament using one of our two desktop fused filament fabrication (FFF) 3D printers: Ultimaker 3 Extended 3D printer (Ultimaker, Geldermalsen, Netherlands) and Moment 3D printer (Moment, Seoul, South Korea). They cost approximately AUD 6,000 and 3,000, respectively. The latter is used to print smaller-sized templates.
Patient recruitment
All patients undergoing postmastectomy autologous breast reconstruction with abdominal wall-based free flaps, regardless of whether immediate or delayed, unilateral or bilateral, and DIEP or MS-TRAM flaps, were recruited from three university-affiliated hospital networks. Exclusion criteria include contraindication to intravenous contrast preventing preoperative investigation with CTA, patient decline and pregnancy. Historical control cases were derived from reconstructions performed at the same institutions over the preceding 12 months (June 2015 – June 2016).
Accuracy of the DIEP template
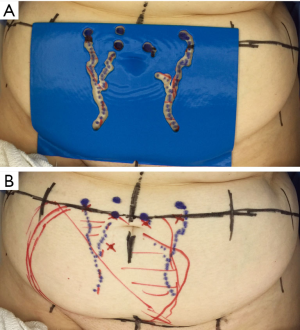
In order to assess the templates accuracy and reliability, perforator distance from the base of umbilicus (horizontal & vertical) was measured using a calibrated calliper. We selected the biggest perforator from each hemi-abdomen. This step was repeated using perforators detected using handheld Doppler probes (33) and reported measurements from the patients CTA (34). Intraoperative measurement of the perforator distances was made using a sterile metal ruler and performed just prior to the flap being disconnected at the DIEA origin. This was achieved with measurements from CTA, with Doppler and with the template applied topically to the abdominal wall directly (see Figure 2).
Utility of the DIEP template: operating time
In order to assess the potential role of the template in improving intraoperative perforator identification, we measured the time taken during suprafascial flap dissection. Recently, Marsh et al. from Chelmsford, UK, described a technique to divide the DIEP flap reconstruction procedure into 101 individual steps (35). As a result, we have measured the time taken during the steps 24 (“lateral raise of flap off rectus fascia with handheld diathermy to just lateral to lateral row perforator”) and 25 [“dissection down to and identification of perforator (match to CT) using bipolar diathermy and/or McIndoe’s dissection scissors”].
Similarly, in order to assess its potential role in improving intramuscular dissection, we have measured the time taken during steps 29 [“subfascial/intramuscular dissection (muscle relaxant or lignocaine) using McIndoe dissecting forceps and bipolar”] and 30 (“submuscular dissection of perforator”) (35).
Since June 2015, at our institution, we have been routinely recording the operating time of the Chelmsford 101 individual steps in free DIEP flap breast reconstructions. As a result, our outcomes from the current study could be accurately correlated to the historical control.
Utility of the DIEP template: surgeon perception surveys
After each operation, both primary and second surgeons and surgical trainees have completed a 5-part survey that assesses their perceived utility of the template on a 10-score visual analogue scale. It consisted of the following questions: “how useful was the device in preoperative marking?”, “how useful was the device in preoperative planning?”, “how useful was the device in intramuscular dissection?”, “did it change your management?” and “would you use it again?”.
Statistical analysis
Perforator distances from the template, handheld Doppler probes, CTA report, and intraoperative findings are recorded using calibrated callipers and sterile rulers. They are rounded to the nearest 1mm. Intraoperative perforator identification time and intramuscular dissection time are recorded using a stopwatch and rounded to the nearest 1 second. The comparative analysis was performed using Stata statistical software package (StataCorp, College Station, TX, USA). The perforator distances were analysed using Kruskal-Wallis equality-of-populations rank test while the surgical times and the survey responses were analysed using the Student’s t-test. A P value of less than 0.05 was accepted as statistically significant.
Results
A total of 20 patient-specific DIEP templates were 3D-printed for 25 consecutive autologous breast reconstructions in 20 patients between July 2016 – June 2017 (12 months). Mean age of the patients was 52 (range: 38–67) and the mean BMI was 27.8 (21–36.4). Immediate reconstruction made up 15 out of 25 flaps (60%), unilateral reconstruction 15 out of 20 cases (75%), and DIEP flaps 18 out of 25 flaps (72%). Historical control consisted of 9 consecutive autologous reconstructions in 7 patients.
3D printing
Each template took mean 18.03 hours to 3D print and used 123.7 g of filament. Given that 750 g of PLA filament costs approximately US$39, mean material cost was less than US$6.50 per template.
Accuracy of the DIEP template
The template accurately identified DIEA perforators and is as accurate as current gold standard imaging modalities: handheld Doppler and CTA. There was no statistical difference between the template and intraoperative measurements (horizontal and vertical distances; P=0.09 and 0.87, respectively). Similarly, there was no statistical difference between the template, handheld Doppler and CTA (horizontal and vertical distances; P=0.42 and 0.74, respectively).
Utility of the DIEP template: operating time
The template had a dissimilar impact on different stages of the operation. Mean intraoperative perforator identification time was significantly reduced by 7.29 minutes (15.07 vs. 22.36 mins; P=0.02). However, there was no statistically significant difference in mean intramuscular dissection time (93.95 vs. 79.62 mins; P=0.34).
Utility of the DIEP template: Surgeon perception survey
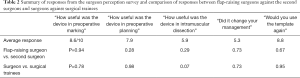
The template was useful for preoperative marking (mean score: 8.6/10) and planning (7.9/10) (Table 2). However, it was not useful for intramuscular dissection (5.9/10) and, as a result, did not influence the clinical management significantly (5.3/10). Encouragingly, the surgeons appeared enthused about its potential and were keen to use the template again (8.8/10). When the responses were compared between flap-raising surgeons and the second surgeons, there was no difference. Similarly, there was no statistical differences between surgeons and surgical trainees. Interestingly, there was a trend in the difference between surgeons and surgical trainees in their perceive utility of the template in intramuscular dissection (4.7 vs. 6.3, P=0.07). However, since the response from trainees was equally low (6.3/10), it is most likely that trainees had similar doubts about its usefulness in intramuscular dissection.
Full table
Clinical outcomes
There was no immediate flap-related postoperative complications. Mean length of stay was 6.4 days (range: 5–8). At 3-month follow-up, there was no reported donor-site morbidity clinically, such as abdominal wall bulge and ventral hernia.
Discussion
In the current prospective case series, we demonstrate our technique of creating a 3D-printed, patient-specific DIEP template, assess its accuracy and utility and illustrate outcomes to our historical control.
Usually, a radiologist would report from CTA the diameter of suitable perforators and their location in horizontal and vertical distances from the umbilicus. This is marked out on the patient by the surgeon preoperatively and confirmed using a handheld Doppler probe. Unsurprisingly, during observation, transcription and interpretation of the report, errors can be introduced and compromise efficiency (36). Thus, Miranda et al. have proposed, in their proof-of-concept study, a method of creating patient-specific 2D DIEP templates using transparent acetate sheets, punch biopsy holes, and coronal images from the CTA (36). However, this technique is rather cumbersome and is significantly susceptible to observation and transcription errors. Moreover, the flat acetate sheet ignores the curved contour of the abdominal wall. To this effect, 3D printing can be useful.
Aided by expiration of key patents, 3D printing, also known as additive manufacturing or rapid prototyping, has become affordable and readily accessible for clinicians in the last decade (23,25-27,37). In comparison to the traditional manufacturing process, 3D printing enables easy customization in a cost-efficient and convenience manner. In plastic and reconstructive surgery, 3D printing appears most useful in preoperative planning, designing intraoperative guidance devices, patient education, and building customized implants (25-27). Using free, open-source softwares (i.e., 3D Slicer, Autodesk MeshMixer, and Cura) and desktop 3D printers (i.e., Ultimaker 3E and Moment), we have produced each template at a material cost of less than AUD 10.
The template accurately identifies the position of DIEA perforators, their intramuscular course and the DIEA pedicle. Furthermore, it improves preoperative marking and planning, leading to statistically quicker intraoperative identification of the perforators. This may be because the template removes the guesswork and interpretation errors from traditional written CTA reports and is more intuitive to apply since they lie accurately on the abdomen. Furthermore, the tactile feedback from templates likely enhances visuospatial understanding of the involved perforator anatomy, leading to more confident dissection (38). However, despite being statistically significant, the reduction of 7.29 minutes may be too small to be clinical significant. Notably, the template appears not useful for intramuscular dissection, which is arguably one of the most technically challenging aspects of DIEP flaps. This is most likely because despite being 3D-printed, clinical information is essentially embossed in 2D on to the template.
3D printing the entire course of a DIEA perforator and its surrounding soft tissues, similar to what Mehta et al. have reported in a proof-of-concept study, may be more useful for intramuscular dissection (39). However, their technique would have been difficult to reproduce for routine clinical application due to software- and hardware-related issues. Using any latest imaging software, it remains difficult to differentiate DIEA perforators from the rectus abdominis in CTA both visually and digitally, especially in patients with physiologically smaller vessels, without significantly increasing the contrast dose and radiation. Using MRA, the image resolution is even poorer (5.0 vs. 0.5 mm slice thickness) since scans have to be performed quickly to prevent motion artefacts. Recent advances in MRA technology can account for motion artefacts without compromising image quality, but they are not yet available widely (40). In contrast to the multi-colour, multi-material, industrial-grade 3D printer used by Mehta et al. that costs in excess of AUD 100,000, our desktop 3D printers (Ultimaker 3E, Moment) can only print in single material, making it difficult to print intramuscular course of DIEA perforators. As a result, most clinicians currently outsource 3D printing at a cost of more than AUD 1,000 per model.
Conclusions
We demonstrate that our 3D-printed, patient-specific DIEP template accurately identifies DIEA perforators, and significantly reduces intraoperative perforator identification time by, albeit 7.29 minutes, and, as a result, it may become a useful tool in preoperative marking and planning.
Acknowledgments
The current work has been accepted and presented at the 9th Congress of World Society for Reconstructive Microsurgery in Seoul, South Korea, and at the Australasian Society of Plastic Surgery’s Plastic Surgery Congress 2017, Gold Coast, Australia.
Funding: None.
Footnote
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-263
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-263). Warren M. Rozen serves as an unpaid Associate Editor of Gland Surgery from Mar 2018 to Feb 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained through Peninsula Health HREC, approval number PH/3DP. The participants gave informed consent before taking part.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265:581-9. [Crossref] [PubMed]
- Jeevan R, Browne JP, Gulliver-Clarke C, et al. Surgical Determinants of Patient-Reported Outcomes following Postmastectomy Reconstruction in Women with Breast Cancer. Plast Reconstr Surg 2017;139:1036e-45e. [Crossref] [PubMed]
- Patel NG, Ramakrishnan V. Microsurgical Tissue Transfer in Breast Reconstruction. Clin Plast Surg 2017;44:345-59. [Crossref] [PubMed]
- Matthews H, Carroll N, Renshaw D, et al. Predictors of satisfaction and quality of life following post-mastectomy breast reconstruction. Psychooncology 2017;26:1860-5. [Crossref] [PubMed]
- Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg 2000;105:930-42. [Crossref] [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Kroll SS. Why autologous tissue? Clin Plast Surg 1998;25:135-43. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Ireton JE, Lakhiani C, Saint-Cyr M. Vascular anatomy of the deep inferior epigastric artery perforator flap: a systematic review. Plast Reconstr Surg 2014;134:810e-21e. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Clavero JA, Masia J, Larranaga J, et al. MDCT in the preoperative planning of abdominal perforator surgery for postmastectomy breast reconstruction. AJR Am J Roentgenol 2008;191:670-6. [Crossref] [PubMed]
- Masia J, Larranaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29-36. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [Crossref] [PubMed]
- Rosson GD, Williams CG, Fishman EK, et al. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery 2007;27:641-6. [Crossref] [PubMed]
- Pratt GF, Rozen WM, Chubb D, et al. Preoperative imaging for perforator flaps in reconstructive surgery: a systematic review of the evidence for current techniques. Ann Plast Surg 2012;69:3-9. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Rozen WM. Comparative study of software techniques for 3D mapping of perforators in deep inferior epigastric artery perforator flap planning. Gland Surg 2016;5:99-106. [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Scott JR, Liu D, Said H, et al. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg 2010;125:446-53. [Crossref] [PubMed]
- Casey WJ 3rd, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [Crossref] [PubMed]
- Sachs EM, Haggerty JS, Cima MJ, et al. Three-dimensional printing techniques. US Patent No. 5,204,055:April 20, 1993.
- Hull CW. Apparatus for production of three-dimensional objects by stereolithography. US Patent No. 4,575,330:March 11, 1986.
- Kamali P, Dean D, Skoracki R, et al. The Current Role of Three-Dimensional (3D) Printing in Plastic Surgery. Plast Reconstr Surg 2016;137:1045-55. [Crossref] [PubMed]
- Chae MP, Rozen WM, McMenamin PG, et al. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25. [Crossref] [PubMed]
- Gerstle TL, Ibrahim AM, Kim PS, et al. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg 2014;133:446-51. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, De-Silva I, et al. Four-Dimensional (4D) Printing: A New Evolution in Computed Tomography-Guided Stereolithographic Modeling. Principles and Application. J Reconstr Microsurg 2015;31:458-63. [Crossref] [PubMed]
- Cabalag MS, Chae MP, Miller GS, et al. Use of three-dimensional printed 'haptic' models for preoperative planning in an Australian plastic surgery unit. ANZ J Surg 2017;87:1057-9. [Crossref] [PubMed]
- Chae MP, Lin F, Spychal RT, et al. 3D-printed haptic "reverse" models for preoperative planning in soft tissue reconstruction: a case report. Microsurgery 2015;35:148-53. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Spychal RT, et al. 3D volumetric analysis for planning breast reconstructive surgery. Breast Cancer Res Treat 2014;146:457-60. [Crossref] [PubMed]
- Rozen WM, Whitaker IS, Stella DL, et al. The radiation exposure of Computed Tomographic Angiography (CTA) in DIEP flap planning: low dose but high impact. J Plast Reconstr Aesthet Surg 2009;62:e654-5. [Crossref] [PubMed]
- Taylor GI, Doyle M, McCarten G. The Doppler probe for planning flaps: anatomical study and clinical applications. Br J Plast Surg 1990;43:1-16. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [Crossref] [PubMed]
- Marsh D, Patel NG, Rozen WM, et al. Three routine free flaps per day in a single operating theatre: principles of a process mapping approach to improving surgical efficiency. Gland Surg 2016;5:107-14. [PubMed]
- Miranda BH, Pywell M, Floyd D. A preoperative marking template for deep inferior epigastric artery perforator flap perforators in breast reconstruction. Arch Plast Surg 2014;41:171-3. [Crossref] [PubMed]
- Hull CW. Method for production of three-dimensional objects by stereolithography. US Patent No. 4, 929,402: May 29, 1990.
- Way TP, Barner KE. Automatic visual to tactile translation--Part II: Evaluation of the TACTile Image Creation System. IEEE Trans Rehabil Eng 1997;5:95-105. [Crossref] [PubMed]
- Mehta S, Byrne N, Karunanithy N, et al. 3D printing provides unrivalled bespoke teaching tools for autologous free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:578-80. [Crossref] [PubMed]
- Ma C, Clifford B, Liu Y, et al. High-resolution dynamic 31 P-MRSI using a low-rank tensor model. Magn Reson Med 2017;78:419-28. [Crossref] [PubMed]


