Preoperative digital mammography imaging in conservative mastectomy and immediate reconstruction
Introduction
Oncoplastic surgery, which combines oncologic and reconstructive surgery, has become increasingly popular (1-3). Conservative mastectomy, including skin sparing mastectomy (SSM), nipple sparing mastectomy (NSM), and skin reducing mastectomy (SRM) (1), is a well-established, validated (4), and widely used procedure for breast cancer treatment; in such cases, immediate breast reconstruction is the current standard (1,4).
Ideally, oncoplastic surgery will provide aesthetically pleasing results while achieving appropriate oncologic safety (5). However, a potential pitfall of these oncoplastic techniques is uncertainty regarding the blood supply to the remaining flaps and the nipple-areola complex (NAC) (2,3). Post-procedural nipple and skin necrosis rates as high as 38% have been reported (5). Patients with a large cup size or a previous history of surgery or radiation are considered high risk for nipple-sparing mastectomies because these factors are associated with even higher rates of complications (2).
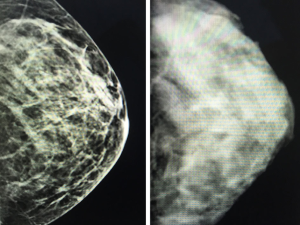
Currently, standard film mammograms do not allow the clear identification and measurement of non-glandular breast tissue coverage. In contrast, digital mammography clearly distinguishes gland tissue density from tegument and fat coverage; accordingly, this preoperative imaging modality can determine the coverage thickness (6,7) (i.e., distance between the breast skin and Cooper’s ligaments surrounding the gland; Figures 1,2). As incision planning, treatment selection, surgical technique, and reconstructive procedures are usually related to the breast volume, tumor characteristics, and surgeons’ and patients’ preferences, preoperative information regarding the breast tissue coverage thickness might highlight the likelihood of post-mastectomy flap issues and assist with the planning process, rather than relying on breast volume alone as a guideline (2).
Methods
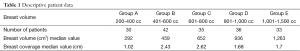
A total of 176 Caucasian women were stratified into five groups according to the Lalardie and Jouglard (8) classification of breast volume. Descriptive statistics regarding data from each group are summarized in Table 1.
Full table
Initially, 200 patients who underwent preoperative digital mammography for conservative mastectomy at our institution between January 2013 and February 2015 were selected randomly. Twenty-four cases were excluded. The exclusion criteria were severe breast asymmetry (>20% difference in size between breasts) and previous breast surgery. A total of 352 preoperative digital mammograms corresponding to the 176 remaining patients were retrospectively reviewed. The subject ages ranged from 33 to 70 years (mean: 49 years).
Breast volume was assessed using the BREAST-V (9), a free simple tool for IOS and Android devices (available from the Apple Store and Google Play Store, respectively) based on a mathematical algorithm that allows estimations of breast volume using direct measurements of three anthropomorphic values. Patients were stratified into groups as described above. All digital mammographic studies were performed on a 3D Selenia Dimensions Full Field Digital Mammograph (Hologic, Bedford, MA, USA). A single evaluator obtained all measurements with OSIRIX Software (available at www.osirix-viewer.com) from DICOM-format digital mammogram files with a lateral medium oblique incidence and angulation between 40° and 50°.
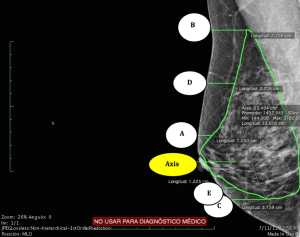
Breast tissue coverage measurements were reported in cm and mm. For each mammogram, measurements were taken at five different points (Figure 3) as follows:
With the axis corresponding to the nipple line:
- A: Parallel to and 2 cm above the nipple (Axis);
- B: Parallel to the superior border of the gland;
- C: Parallel to the inferior border of the gland;
- D: Parallel to and at a midpoint between A and B;
- E: Parallel to and at a midpoint between the Axis and C.
For each image, average tissue coverage was obtained and correlated with the corresponding breast volume group (A to E; Table 1).
Statistical analyses were performed using R software (version 2.14.2) (10). As 95% of our sample fell within approximately two standard deviations of the mean, we obtained mean tissue cover thickness measures to establish reference intervals for our breast tissue cover classification. As a result, breast tissue coverage was coded as poor (type 1), medium (type 2), and good (type 3) according to the mean standard deviations of the overall values (Table 2).
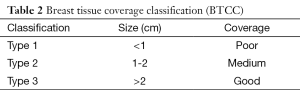
Full table
Results
Differences between two directly consecutive breast volume groups were not statistically significant; however, there was a trend toward a flap thickness increase when comparing groups with greater differences in breast volume. Breast tissue coverage varied from 0.2 to 4.4 mm, with an average of 1.952 cm (Table 1). The median values for measurements A, B, C, D, and E were 1.02, 2.43, 2.62, 1.68, and 1.71 cm, respectively.
In our analysis of breast tissue coverage and breast volume (Table 3), we observed the following. In group A (30 patients with breast volumes of 200-400 cm3), 19 patients had tissue coverage of 0.1-1 cm, 9 had tissue coverage of 1.1-2 cm, and 2 had tissue coverage exceeding 2 cm. In group B (42 patients with breast volumes of 401-600 cm3), 12 patients had tissue coverage of 0.1-1 cm, 20 had tissue coverage of 1.1-2 cm, and 10 had tissue coverage exceeding 2 cm. In group C (35 patients with breast volumes of 601-800 cm3), 8 patients had tissue coverage of 0.1-1 cm, 15 had tissue coverage of 1.1-2 cm, and 12 had tissue coverage exceeding 2 cm. In group D (36 patients with breast volumes of 801-1,000 cm3), 6 patients had tissue coverage of 0.1-1 cm, 14 had tissue coverage of 1.1-2 cm, and 16 had tissue coverage exceeding 2 cm. In group E (33 patients with breast volumes of 1,001-1,500 cm3) 7 patients had tissue coverage of 0.1-1 cm, 13 had tissue coverage of 1.1-2 cm, and 13 had tissue coverage exceeding 2 cm.
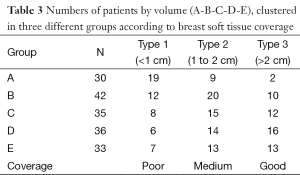
Full table
Discussion
To our knowledge, no reports have previously addressed the relationship between breast tissue coverage and breast volume. However, adequate fat tissue coverage thickness is one of the most important independent factors in immediate breast reconstruction and flap survival (11-13). Anatomically, the vascular network that ensures flap survival and NAC runs between Cooper’s ligaments and the skin (14). Compression of this vascular network by implant insertion, surgical damage, tissue tension at closure, or extremely thin flaps might endanger vascularization, and such events have been shown to cause tissue damage in the distal parts of flaps (8-21). Consideration must therefore be given to this preoperative breast tissue coverage measure as an important factor in immediate reconstruction.
Preoperative evaluation of gland coverage can help to predict the viability of the remaining flaps after conservative mastectomies and to select the optimal immediate reconstructive procedure to diminish post-operative coverage complications. Additionally, the use of surgical materials may be evaluated according to this coverage measure. According to our classification, for patients in the Poor coverage group (type 1), it would be helpful to add supplementary coverage for the reconstruction, such as ADM, retropectoral implant placement, and delayed fat grafting. In the medium coverage group (type 2), a 2-stage reconstruction should be suggested to avoid tension at the flap closure, whereas in the good coverage group (type 3), single-stage reconstruction with implants could be performed.
One of the most important factors for vascularization of the remaining post-mastectomy flaps is preservation of the skin perforators and flap thickness (11,12,17,18). The remaining skin flap thickness after gland resection during conservative mastectomy plays an important role in flap integrity and NAC vitality. Cooper’s ligaments separate the mammary gland from the superficial fat and skin tissue layers that contain the vascular plexus, of which the mastectomy flaps are composed (13). The vascularization and, therefore, the viability of the remaining flaps may be compromised after gland resection if this flap tissue coverage is too thin and/or closure tension is forced. Preoperative information regarding this tissue coverage is therefore of the utmost importance to avoid complications associated with immediate reconstruction procedures (11,16,17).
The selection of mastectomy and reconstruction procedures should be made jointly by the oncologic and plastic surgeon based on objective pre-operative information (12,18,19). In this study, we observed that breast tissue coverage and breast volume are independent factors (Table 1). This finding suggests that a preoperative measurement of the breast tissue coverage thickness is important for surgical decisions.
For large breasts, conservative mastectomy is usually designed according to the Wise pattern for skin reduction, shape, and projection. This procedure is considered suitable for single-stage reconstruction with implants (4). Regardless of breast volume, however, a preoperative evaluation of tissue coverage is crucial for surgical planning by both the oncologic and plastic surgeon as this factor is directly related to flap and NAC ischemia/necrosis. Thin flaps can lead to ischemic complications following mastectomies and reconstructive procedures (11,17). Preoperative digital mammography is therefore potentially useful not only for tumor detection, but as an objective tool for predicts the resulting flap thickness, thus improving patient safety.
Flap damage after mastectomy is a serious complication during immediate breast reconstruction (22-24). Preoperative breast tissue coverage and flap thickness evaluations via digital mammography should be considered during surgical planning, and the proposed classification may help to identify patients at high risk for flap ischemia and necrosis. Digital mammography offers the possibility of preoperative measurements and better predictions of flap thickness and vitality after mastectomy (6,7), thus improving patient safety (13,14,20,25,26).
Based on the obtained range of coverage values, we propose a 3-stage breast tissue coverage classification (BTCC) as follows: type 1, ≤1 cm (poor coverage); type 2, 1-2 cm (medium coverage); and type 3, >2 cm (good coverage; Table 2). This classification may inform the rational use of materials for individual patients rather than according to breast volume, surgeon’s experience, or comfort (21,27). As a result, preoperative communication between the reconstructive and oncologic surgeons regarding the incision choice and integumentary preservation according to digital mammogram findings might lead to improved outcomes with a decreased rate of complications.
This study has generated normative data for breast tissue coverage measurements in different breast volumes to provide three thickness classification levels. This information may be useful as a reference for future investigations of various breast surgical procedures and for the rational use of materials in conservative mastectomies and immediate reconstruction. Our study is limited, however, by the lack of validation of the BREAST-V tool against standard 3D virtual techniques; nevertheless, this tool underwent a strict development process that included internal and cross-validation of the model to verify the reliability of the algorithm (9,19). A comparison of the predictive performances of BREAST-V with a previous published formula demonstrated higher accuracy when evaluating breast volumes on new breasts (i.e., those not used to derive the formula). These considerations highlight the reliability of this tool.
Conclusions
This report provides a complete data bank of normative non-glandular breast tissue coverage measurements from digital mammograms across a wide range of breast volumes, and suggests that breast volume and flap thickness are independent factors; in other words, large breasts, (C, D, and E volume categories) can have poor tissue coverage, whereas small breasts (A and B categories) can have good tissue coverage.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [PubMed]
- Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [PubMed]
- Staub G, Fitoussi A, Falcou MC, et al. Breast cancer surgery: use of mammaplasty. Results. Series of 298 cases. Ann Chir Plast Esthet 2008;53:124-34. [PubMed]
- della Rovere GQ, Nava M, Bonomi R, et al. Skin-reducing mastectomy with breast reconstruction and sub-pectoral implants. J Plast Reconstr Aesthet Surg 2008;61:1303-8. [PubMed]
- Longo B, Farcomeni A, Ferri G, et al. The BREAST-V: a unifying predictive formula for volume assessment in small, medium, and large breasts. Plast Reconstr Surg 2013;132:1e-7e. [PubMed]
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007;244:356-78. [PubMed]
- Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology 2007;244:672-91. [PubMed]
- Lalardie JP, Jouglard P. Chirurgie plastique du Sein. Paris: Masson, 1974.
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [PubMed]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available online: http://www.R-project.org/
- Nahabedian MY. Overview of perforator imaging and flap perfusion technologies. Clin Plast Surg 2011;38:165-74. [PubMed]
- Seitz IA, Nixon AT, Friedewald SM, et al. "NACsomes": A new classification system of the blood supply to the nipple areola complex (NAC) based on diagnostic breast MRI exams. J Plast Reconstr Aesthet Surg 2015;68:792-9. [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [PubMed]
- Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg 2006;117:1083-90. [PubMed]
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [PubMed]
- Heywang-Köbrunner SH, Hacker A, Sedlacek S. Magnetic resonance imaging: the evolution of breast imaging. Breast 2013;22 Suppl 2:S77-82. [PubMed]
- Cunningham L. The anatomy of the arteries and veins of the breast. J Surg Oncol 1977;9:71-85. [PubMed]
- Longo B, Campanale A, Santanelli di Pompeo F. Nipple-areola complex cutaneous sensitivity: a systematic approach to classification and breast volume. J Plast Reconstr Aesthet Surg 2014;67:1630-6. [PubMed]
- Murray JD, Jones GE, Elwood ET, et al. Laser angiography as a predictor of mastectomy flap necrosis after breast reconstruction. Plast Reconstr Surg 2012;129:1017e-18e. [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [PubMed]
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013;131:969-84. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg 2010;126:1460-71. [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [PubMed]
- Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 2004;113:877-81. [PubMed]
- Chu CK, Carlson GW. Techniques and Outcomes of Nipple Sparing Mastectomy in the Surgical Management of Breast Cancer. Curr Breast Cancer Rep 2013;5:118-24.