Increasing options in autologous microsurgical breast reconstruction: four free flaps for ‘stacked’ bilateral breast reconstruction
Introduction
Autologous breast reconstruction is often considered a preference to alloplastic options, given that a more natural shape and feel can be achieved, as well as the creation of a breast with ptosis and volume. The deep inferior epigastric artery (DIEA) perforator flap is felt to be the most ideal option, with second tier options available that include the transverse upper gracilis (TUG), lumbar and latissimus dorsi flaps (1-5). There are cases, however, where even a deep inferior epigastric perforator (DIEP) flap cannot provide the volume of autologous tissue required for a unilateral breast reconstruction, and as a consequence, the concept of simus dor bilateral DIEP flaps was developed, in which hemi-abdominal flaps are raised on each DIEA, and both flaps transferred to the chest recipient site.
While the terminology has been confusing, with terms used to mean various flap configurations, the terms ‘stacked’, ‘double-pedicled’ or ‘bipedicled’ have each been used to describe inclusion of the entire abdominal pannus on two pedicles, transferred to create a single breast reconstruction. Prior to the design of DIEP flaps being introduced into clinic practice, the abdominal wall was used in a bipedicled fashion in the way of bipedicled or stacked transverse rectus abdominis myocutaneous (TRAM) flaps (6). Bipedicled TRAM flaps were achieved through a pedicled TRAM flap (superior epigastric pedicle), supplemented with the use of a microsurgical anastomosis to the DIEA or vein, or with the use of both superior and inferior pedicles (6). Arnez et al. described the bipedicled free TRAM flap, anastomosed onto both the thoracodorsal and serratus anterior branches, which was in particular described as useful for cases with midline abdominal scars that required larger volume reconstructions (7). Donor morbidity associated with sacrificing both rectus abdominis muscles was considered unacceptable, leading to diminishing use of both rectus muscles in the form of both TRAM flaps (8,9), however, microsurgical augmentation of a unilateral TRAM flap was widely described in a range of vascular configurations, with different pedicle arrangements, cross-over anastomoses and retro-grade vascular loops have all been described (10-16).
With the development of the DIEP flap (1-5), the use of ‘stacked’ or ‘double-pedicled’ DIEP flaps was reintroduced by the current senior author of this paper (17). The stacked DIEP flap concept is of particular benefit for thinner patients and those with midline abdominal scars. The use of stacked DIEP flaps has been successfully reported now in a range of clinical series, and with a range of classifications for pedicle arrangements described (17-25). Where stacked DIEP flaps are not possible, the superficial inferior epigastric artery (SIEA) has been used as a secondary pedicle (19), and bilateral profunda artery perforator flaps stacked have been used (26).
In cases of bilateral breast reconstruction, stacked flaps may be required to achieve volume replacement; however options have not been described. Herein we demonstrate the utility of using stacked flaps for bilateral breast reconstruction, using one DIEP flap per side stacked with one TUG flap for the reconstruction of each breast.
Case presentation
A 49-year-old woman, with BRCA1 mutation, attended the multidisciplinary risk-reducing clinic, with a decision from the medical team and patient to undertake bilateral risk-reducing mastectomies, and immediate breast reconstruction. The patient had a strong preference for autologous reconstruction alone, and due to a paucity of abdominal tissue, a decision was made to use stacked flaps bilaterally: with a DIEP flap and TUG flap suitable for reconstruction of each side.
The patient was well, with no known comorbidities, a non-smoker and no medications.
Surgical technique
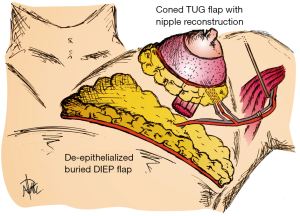
Flap design was planned in a manner to achieve maximal projection and primary nipple reconstruction. This was able to be achieved by using the DIEP flap de-epithelialized and completely buried, with the flap orientated with the pedicle on its superficial surface, and the TUG flap lying superficially with its skin paddle used for nipple reconstruction and able to be monitored clinically (see schematic in Figure 1).
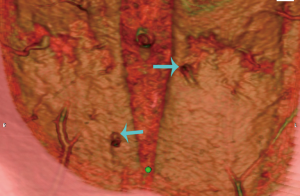
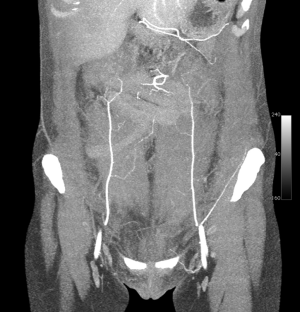
A preoperative computed tomographic angiogram (CTA) of the abdominal wall vasculature was used to delineate the optimal perforators for DIEP flap harvest (see Figure 2). The CTA was able to also delineate the recipient pedicle for the TUG flap. This is highlighted in Figure 3, in which a type 1 DIEA was identified bilaterally, with the distal end of the DIEA thus selected as the recipient vessels for the TUG flap on each side.
Given the operative complexity, three concurrent surgical teams were operating, utilized in the following manner:
- Stage 1—one team harvesting the first TUG flap, one team harvesting the first DIEP flap and one team performing the first mastectomy;
- Stage 2—one team closing the first TUG flap donor site, one team on the side table performing an intra-flap anastomosis and flap shaping (each DIEP and TUG flap were anastomosed in series on a side table), and one team performing the second mastectomy;
- Stage 3—one team raising the second TUG flap, one team raising the second DIEP flap, and one team performing the microsurgical anastomoses at the first chest wall recipient site;
- Stage 4—one team closing the second TUG flap donor site, one team closing the abdominal donor site, and one team on the side table performing an intra-flap anastomosis and flap shaping of the second DIEP and TUG flaps;
- Stage 5—one team completing donor site closure and dressings, one team completing inset and dressings of the first breast reconstruction, and one team performing microsurgical anastomoses and flap inset of the second breast reconstruction.
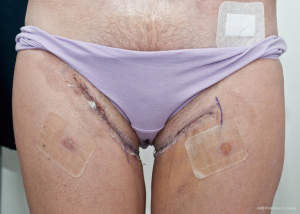
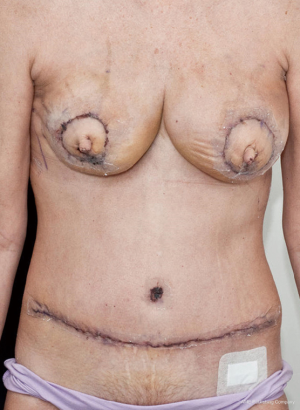
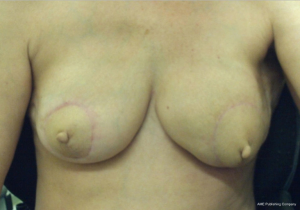
The case proceeded uneventfully, with a single perforator DIEP flap raised on each side (see CTA in Figure 2), and the thoracodorsal vessels used as recipient vessels bilaterally. For one side, the pedicle length necessitated a vein graft for reach, with a long saphenous vein tributary from the thigh donor site used (and thus no additional morbidity). The duration of the case was just under 8 hours, and the patient had an uneventful early perioperative and immediate postoperative course, discharged home on day 7 post-operatively (see Figure 4). Of note, the patient was given preoperative clexane for venous thrombo-prophylaxis, and this was continued for 1 week post-operatively with the concurrent use of graded compression stockings, until full mobilization was achieved. There were no flap-related complications, and the donor sites healed unremarkably (see Figures 4-6). The aesthetic result at 3 months postoperatively is shown in Figure 7.
There was, however, a significant complication that arose on day 14 postoperatively. The patient presented on the 14th postoperative day to the emergency department with a dense hemiplegia and aphasia consistent with a cerebrovascular stroke. Investigations were performed, including a carotid Doppler which demonstrated a right carotid free floating thrombus, and both CT and MRI which demonstrated a right-sided ischaemic stroke, mass effect with midline shift and decreased ventricular size. The patient underwent immediate transfer to a neurosurgical centre, where she was taken to theatre for decompressive craniectomy. She responded well, with improvement in clinical and imaging findings, and discharge 7 days later. With ongoing neurologic rehab 2 months later, the patient has shown resolution of her aphasias/dysphasia, but an ongoing hemiplegia. The case was discussed within the neurosurgical and anaesthetic department meetings, and the cause for the stroke is unknown. No patient factors for the carotid thrombosis have been identified despite haematologic screening, no hyperextension of the neck during the anaesthetic was observed but may have contributed, and while a prolonged operation may be theoretically contributory, the 8 hours for this case was not clearly considered a factor.
Discussion
The use of stacked abdominal flaps has been a widely used and successful addition to the armamentarium of autologous breast reconstruction options in unilateral reconstruction cases. In bilateral cases, the options are much more limited. We present an option, in which stacked flaps are used for the reconstruction of each breast, with the TUG flap used to augment bilateral DIEP flaps. Our case demonstrates the relative efficiency of such an approach, and the aesthetic outcome able to be achieved in a patient with paucity of abdominal volume.
There are several key factors that are essential to achieve success in this approach. The first is the use of preoperative CTA. The preoperative CTA can highlight the optimal perforator for flap harvest, reducing harvest times and ensuring that the case suitable, and optimized. The ability of CTA to achieve these ends with accuracy has been demonstrated in multiple previous studies (27-32), highlighting a high degree of specificity and sensitivity, and showing improvements in flap-related outcomes and operative times. In addition to CTA, the use of three surgical teams concurrently operating is essential for operative efficiency. In the five stages highlighted, there is never a team not contributing to the case, and we would advise this to ensure that such a case does not encroach upon excessive operative times.
While the aesthetic and reconstructive outcomes were all achieved, the devastating complication encountered is a reminder as to the risks of any surgery. The consent process is paramount, particularly in the case of risk-reduction surgery, and it is essential that each patient weigh-up the risks in electing to proceed in any breast reconstructive case. While no factors were implicated in the causality or even to be contributory to this outcome in our case, diligence in case selection and prophylactic measures for all complications are essential.
Conclusions
We describe the use of stacked free flaps for bilateral breast reconstruction, using one DIEP flap per side stacked with a TUG flap from each side. The technique offers a further option in microsurgical breast reconstruction for patients in whom there is a paucity of abdominal tissue for reconstruction and in whom prosthetics are not considered an option.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [PubMed]
- Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg 1997;50:322-30. [PubMed]
- Futter CM, Webster MH, Hagen S, et al. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg 2000;53:578-83. [PubMed]
- Nahabedian MY, Dooley W, Singh N, et al. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: the role of muscle preservation. Plast Reconstr Surg 2002;109:91-101. [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [PubMed]
- Spear SL, Travaglino-Parda RL, Stefan MM. The stacked transverse rectus abdominis musculocutaneous flap revisited in breast reconstruction. Ann Plast Surg 1994;32:565-71. [PubMed]
- Arnez ZM, Scamp T. The bipedicled free TRAM flap. Br J Plast Surg 1992;45:214-8. [PubMed]
- Simon AM, Bouwense CL, McMillan S, et al. Comparison of unipedicled and bipedicled TRAM flap breast reconstructions: assessment of physical function and patient satisfaction. Plast Reconstr Surg 2004;113:136-40. [PubMed]
- Jensen JA. Is double pedicle TRAM flap reconstruction of a single breast within the standard of care? Plast Reconstr Surg 1997;100:1592-3. [PubMed]
- Pennington DG, Nettle WJ, Lam P. Microvascular augmentation of the blood supply of the contralateral side of the free transverse rectus abdominis musculocutaneous flap. Ann Plast Surg 1993;31:123-6; discussion 126-7. [PubMed]
- Semple JL. Retrograde microvascular augmentation (turbocharging) of a single-pedicle TRAM flap through a deep inferior epigastric arterial and venous loop. Plast Reconstr Surg 1994;93:109-17. [PubMed]
- Blondeel PN, Boeckx WD. Refinements in free flap breast reconstruction: the free bilateral deep inferior epigastric perforator flap anastomosed to the internal mammary artery. Br J Plast Surg 1994;47:495-501. [PubMed]
- Lam TC, Sellars GD. Free perforator crossover TRAM flap for breast reconstruction. Ann Plast Surg 2003;50:126-31. [PubMed]
- Tseng CY, Lang PO, Cipriani NA, et al. Pedicle preservation technique for arterial and venous turbocharging of free DIEP and muscle-sparing TRAM flaps. Plast Reconstr Surg 2007;120:851-4. [PubMed]
- Agarwal JP, Gottlieb LJ. Double pedicle deep inferior epigastric perforator/muscle-sparing TRAM flaps for unilateral breast reconstruction. Ann Plast Surg 2007;58:359-63. [PubMed]
- Schoeller T, Wechselberger G, Roger J, et al. Management of infraumbilical vertical scars in DIEP-flaps by crossover anastomosis. J Plast Reconstr Aesthet Surg 2007;60:524-8. [PubMed]
- Ali RS, Garrido A, Ramakrishnan V. Stacked free hemi-DIEP flaps: a method of autologous breast reconstruction in a patient with midline abdominal scarring. Br J Plast Surg 2002;55:351-3. [PubMed]
- Kronowitz SJ, Robb GL, Youssef A, et al. Optimizing autologous breast reconstruction in thin patients. Plast Reconstr Surg 2003;112:1768-78. [PubMed]
- Figus A, Fioramonti P, Ramakrishnan V. Stacked free SIEA/DIEP flap for unilateral breast reconstruction in a thin patient with an abdominal vertical midline scar. J Reconstr Microsurg 2007;23:523-5. [PubMed]
- Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg 2007;60:904-12. [PubMed]
- Rabey NG, Erel E, Malata CM. Double-pedicled abdominal free flap using an entirely new microvascular combination of DIEP and SIEA vascular pedicles for unilateral breast reconstruction: a novel addition to the Hamdi classification. Plast Reconstr Surg 2012;130:767e-9e. [PubMed]
- DellaCroce FJ, Sullivan SK, Trahan C. Stacked deep inferior epigastric perforator flap breast reconstruction: a review of 110 flaps in 55 cases over 3 years. Plast Reconstr Surg 2011;127:1093-9. [PubMed]
- Beahm EK, Walton RL. The efficacy of bilateral lower abdominal free flaps for unilateral breast reconstruction. Plast Reconstr Surg 2007;120:41-54. [PubMed]
- Chan RK, Przylecki W, Guo L, et al. Case report. The use of both antegrade and retrograde internal mammary vessels in a folded, stacked deep inferior epigastric artery perforator flap. Eplasty 2010;10:e32. [PubMed]
- Murray A, Wasiak J, Rozen WM, et al. Stacked abdominal flap for unilateral breast reconstruction. J Reconstr Microsurg 2015;31:179-86. [PubMed]
- Blechman KM, Broer PN, Tanna N, et al. Stacked profunda artery perforator flaps for unilateral breast reconstruction: a case report. J Reconstr Microsurg 2013;29:631-4. [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg 2008;122:1003-9. [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. The effect of anterior abdominal wall scars on the vascular anatomy of the abdominal wall: A cadaveric and clinical study with clinical implications. Clin Anat 2009;22:815-22. [PubMed]
- Rozen WM, Ashton MW, Grinsell D. The branching pattern of the deep inferior epigastric artery revisited in-vivo: a new classification based on CT angiography. Clin Anat 2010;23:87-92. [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [PubMed]
- Katz RD, Manahan M, Rad AN, et al. Classification schema for anatomic variations of the inferior epigastric vasculature evaluated by abdominal CT angiograms for breast reconstruction. Microsurgery 2010;30:593-602. [PubMed]