
Correlation between sonographic features and pathological findings of cervical lymph node metastasis of differentiated thyroid carcinoma
Introduction
The incidence of lateral cervical lymph node metastasis in patients with thyroid cancer can reach 37.3%. Studies have shown that patients with lymph node metastasis are more likely to have a relapse and experience metastasis, which can adversely affect their long-term survival rate (1-3). The sonographic features of metastatic lymph nodes in differentiated thyroid carcinoma are more specific than those of other organ tumors, and mainly include hyperechoic changes, microcalcification, and cystic changes (4,5). Ultrasonography is the first choice for the evaluation of cervical lymph node metastasis of thyroid cancer. However, the sensitivity and specificity of ultrasonography in the diagnosis of lateral and central cervical lymph node metastasis are 84–94% and 80–98%, and 40–51% and 71–78%, respectively. This is important because the presence or absence of lymph node metastasis seriously affects the choice of treatment plan (especially the surgical method) (6-8). The purpose of this study was to investigate the relationship between the sonographic features and the pathological findings of cervical metastatic lymph nodes in differentiated thyroid carcinoma, so as to better understand and fully evaluate the cervical lymph node metastasis of thyroid cancer. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-21-253).
Methods
General data
A total of 49 patients, 15 males and 34 females, with a mean age of 48±9.2 years (range, 19–81 years) who had suspicious metastatic lymph nodes from thyroid cancer according to intraoperative ultrasonography and who had been prepared for subtotal thyroidectomy or total thyroidectomy and lateral and central cervical lymph node dissection from October 2019 to December 2019 in our hospital were selected. All patients underwent comprehensive ultrasound examination before operation to evaluate the thyroid lesions, and central and lateral cervical lymph nodes. Apart from a few small suspicious lymph nodes that were marked by preoperative body surface examination, all the other lymph nodes dissected were evaluated, marked, and grouped by intraoperative ultrasound in vitro. Two professional sonographers with the operational experiences more than 5-year made the diagnosis from the ultrasonic images. If their opinions diverged, the ultrasonic images would be submitted to the superior doctors for a further diagnostic decision. Results of routine pathological examination were considered as the gold standard for making the diagnosis. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Affiliated Hospital of Soochow University (NO. 114) and informed consent was taken from all the patients.
Instruments and methods
An Esaote MyLab Alpha portable ultrasonic diagnostic instrument (model Sl3116) with a frequency of 10–22 MHz was applied to check the lymph nodes.
Preoperative ultrasonography
Patients were placed in the supine position with their neck fully exposed. Routine ultrasound examination was performed on the thyroid and cervical lymph nodes. The frequency, depth, focus, gain, and dynamic range of the sound velocity probe were adjusted as appropriate. The images of thyroid and cervical lymph nodes were recorded and stored. If necessary, the suspicious lymph nodes in the lateral cervical region were localized on the body surface before operation.
Intraoperative ultrasonography
The dissected tissues containing lymph nodes were placed between the ultrasound pads, and the space between the ultrasound pad and the lymph nodes was filled with the appropriate amount of normal saline. The SL3116 linear array probe was used to scan, explore, and record all ultrasound
Statistical analysis
SPSS v. 22.0 software (IBM Corp.) was used for statistical analysis. The measurement data are expressed as mean ± standard deviation (x±s). Chi square test and Fisher exact test were used for counting data. The difference was considered to be statistically significant at P value <0.05.
Results
A total of 281 lymph nodes were detected by intraoperative ultrasound, and 120 suspicious metastatic lymph nodes were screened out in vitro. The suspicious metastatic lymph nodes in the lateral and central regions of the neck showed significantly different sonographic signs from those of the normal lymph nodes in group A (27.27–44.29% vs. 0–2.78%; P=0.000, P=0.000, respectively), group B (24.29–32.73% vs. 2.38–8.33%; P=0.000, P=0.001, respectively), and group C (10.91–20.00% vs. 0–5.95%, P=0.008, P=0.006, respectively). There was no significant difference in the sonographic signs between normal lymph nodes and the lymph nodes in group D (4.29% vs. 1.19%; P=0.330). There was a significant difference between differentiating the normal lymph nodes from metastatic lymph nodes in the lateral cervical region versus the central region (7.14–29.09% vs. 88.89–90.48%; P=0.000, P=0.000, respectively) in group E. However, the misdiagnosis rate of group E was higher in the central cervical region than in lateral cervical region (29.09% vs. 7.14%, P=0.009; Table 1).
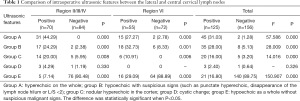
Full table
The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of intraoperative ultrasound in the diagnosis of lateral and central lymph nodes were 89.04% vs. 82.98%, 93.83% vs. 80.00%, 90.97% vs. 81.10%, 92.86% vs. 70.91%, and 90.48% vs. 88.89%, respectively (Table 2).

Full table
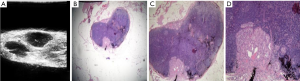
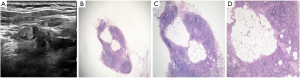
The pathological microscopic features of metastatic lymph nodes in each group were the following. In the lymph nodes in group A, there were diffuse thyroid follicular structures, which were composed of atypical thyroid follicular epithelial cells; in group C, there were thyroid follicles distributed in clusters in the lymph nodes; and in group B and E there were atypical thyroid follicular epithelial cells distributed in clusters or diffused in the metastatic lymph nodes, with or without papillary structures. There were significant differences in the ultrasonic features between the lymph nodes in group A, C, B, and E, and the normal lymph nodes (95.74% vs. 0.00% vs.4.30%, P=0.000; 80% vs. 0.00% vs. 20%, P=0.000; 0.00% vs. 81.40% vs. 18.60%, P=0.000; 0.00% vs. 13.04% vs. 86.96%, P=0.000; respectively). Necrosis and liquefaction were observed in the lymph nodes in group D, and there was significant difference in the ultrasonic features between the lymph nodes in group D and the normal lymph nodes (0.00% vs. 0.00% vs. 100%, P=0.094; Table 3; Figures 1-4).
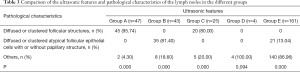
Full table
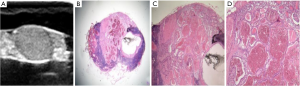
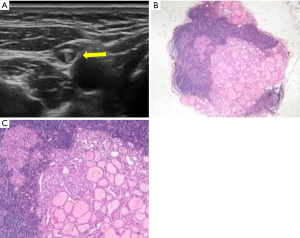
Discussion
Differentiated thyroid carcinoma mainly includes papillary thyroid carcinoma and follicular carcinoma, with good prognosis. The 10-year survival rate of papillary thyroid carcinoma can reach more than 90% and the prognosis of follicular carcinoma is slightly worse than that of papillary carcinoma (9-11). However, studies have shown that the recurrence rate of thyroid cancer with lymph node metastasis is higher and may have a certain impact on the long-term survival rate. Therefore, the full evaluation of cervical lymph node metastasis in patients with thyroid cancer before operation is of great significance for the selection of surgical methods and prognosis (12-17).
Conventional ultrasonography is the preferred imaging examination method for thyroid-related diseases (12) and has high diagnostic efficiency for metastatic lymph nodes in the lateral cervical region, but the detection rate for lymph node metastasis in the central neck is not high. This study showed that the sensitivity, specificity, and accuracy of intraoperative ultrasound in the diagnosis of metastatic lymph nodes in the lateral and central regions were 89.04% vs. 82.98%, 93.83% vs. 80.00%, and 90.97% vs. 81.10%, respectively, which were higher than those reported previously, especially in the central region (6,7,18,19). We analyzed the possible reasons for this discrepancy: (I) the metastatic lymph nodes in central neck are easily missed in routine ultrasound examination on the body surface due to their deep location and small volume, while the detection rate of the metastatic lymph nodes in central neck are greatly improved by intraoperative ultrasound; (II) the influence of intratracheal air increases the difficulty of surface ultrasound detection. Compared with the metastatic lymph nodes from other systems, metastatic lymph nodes of thyroid cancer often have their own typical sonographic features: microcalcification, hyperechoic mass or whole hyperechoic changes, cystic changes, etc. (4,5,20). The results of this study showed that features A, B, and C, 3 conventional ultrasonographic features in the prediction of lateral and central lymph node metastasis, were statistically significant, but feature D showed no statistical significance in the prediction of lateral and central lymph node metastasis (P=0.330). The reason for this may be that the total number of lymph nodes with feature D is too small, which might have resulted in serious bias in the statistical analysis. In addition, the misdiagnosis rate of normal lymph nodes based on feature E was higher in the central region than in the lateral cervical region (29.09% vs. 7.14%, P=0.009), indicating that hypoechoic metastatic lymph nodes were more likely to be misdiagnosed, especially in the prediction of central lymph nodes. When Hashimoto’s thyroiditis is complicated, central cervical lymph nodes with reactive hyperplasia can be easily misdiagnosed as metastatic lymph nodes when the shape is almost round (21).
The results showed that the unique ultrasonographic features of thyroid metastatic lymph nodes were closely related to their pathological structures: under microscopy, the overall hyperechoic metastatic lymph nodes showed diffuse follicular structure under microscope; while the nodular hyperechoic metastatic lymph nodes in the cortex showed clustered follicular structures. We further analyzed the reasons for this: the echo level of tissues or lesions on ultrasound images is related to the number of acoustic interfaces and the acoustic impedance difference of media on both sides of the interface (22).
In the follicular structure of metastatic lymph nodes, the epithelium of the follicle and the colloid in the follicular cavity form acoustic interfaces with a large acoustic impedance difference. When a large number of follicles are densely present, a large number of acoustic interfaces are formed, resulting in high echo changes. In group A, 2 cases were misdiagnosed because one of them was a hyperechoic parathyroid gland and the other was residual thyroid tissue. In group C, 5 lesions of the same patient were misdiagnosed as metastatic lymph nodes when they were actually fat cells in clusters under microscopy. Fat cells have a similar diameter to that of follicles and contain lipid droplets, which can also form acoustic interface. Therefore, when nodular hyperechogenicity appears in cervical lymph nodes of patients with thyroid cancer, it is necessary to be careful to identify whether there is focal steatosis in the lymph nodes. Heterotypic follicular epithelial cells clustered or diffusely distributed with or without papillary structures were observed under microscopy in group B and group E. The components were relatively simple, and there were no or very few acoustic interfaces with large acoustic impedance difference. Therefore, hypoechoic changes were observed. The echo level was similar to that of the lymph node cortex and was difficult to distinguish. Therefore, due to the lack of other typical signs, the lymph nodes in group E were easily misdiagnosed. If necessary, fine-needle aspiration cytology guided by ultrasound should be performed to avoid misdiagnosis.
The limitations of this study are that no quantitative analysis of the follicular structure of metastatic lymph nodes under microscopy was performed. Furthermore, there was the limited sample size, and we failed to study the cystic changes of metastatic lymph nodes more deeply.
In summary, the unique sonographic features of metastatic lymph nodes in differentiated thyroid carcinoma are closely related to the content of follicular structure in lymph nodes. A correct understanding of these features is helpful to improving the diagnostic rate of conventional ultrasonography and to reducing the incidences of misdiagnosis and missed diagnoses.
Acknowledgments
Funding: Six Top Talent Projects in Jiangsu Province (WSW-056).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-21-253
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-21-253
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-21-253). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Affiliated Hospital of Soochow University, (NO. 114) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004;135:139-48. [Crossref] [PubMed]
- Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003;134:946-54; discussion 54-5. [Crossref] [PubMed]
- Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg 2006;141:489-94; discussion 94-6. [Crossref] [PubMed]
- Gor DM, Langer JE, Loevner LA. Imaging of cervical lymph nodes in head and neck cancer: the basics. Radiol Clin North Am 2006;44:101-10. viii. [Crossref] [PubMed]
- Leboulleux S, Girard E, Rose M, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 2007;92:3590-4. [Crossref] [PubMed]
- Wu LM, Gu HY, Qu XH, et al. The accuracy of ultrasonography in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: A meta-analysis. Eur J Radiol 2012;81:1798-805. [Crossref] [PubMed]
- Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 2011;121:487-91. [Crossref] [PubMed]
- Park JS, Son KR, Na DG, et al. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol 2009;192:66-72.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587-95. [Crossref] [PubMed]
- Spinelli C, Tognetti F, Strambi S, et al. Cervical Lymph Node Metastases of Papillary Thyroid Carcinoma, in the Central and Lateral Compartments, in Children and Adolescents: Predictive Factors. World J Surg 2018;42:2444-53. [Crossref] [PubMed]
- Shin LK, Olcott EW, Jeffrey RB, et al. Sonographic evaluation of cervical lymph nodes in papillary thyroid cancer. Ultrasound Q 2013;29:25-32. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Jikuzono T, et al. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg 2007;31:838-48. [Crossref] [PubMed]
- Barbosa MP, Momesso D, Bulzico DA, et al. Metastatic lymph node characteristics as predictors of recurrence/persistence in the neck and distant metastases in differentiated thyroid cancer. Arch Endocrinol Metab 2017;61:584-9. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Roh JL, Park JY, Kim JM, et al. Use of preoperative ultrasonography as guidance for neck dissection in patients with papillary thyroid carcinoma. J Surg Oncol 2009;99:28-31. [Crossref] [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg 2005;29:917-20. [Crossref] [PubMed]
- Ahn JE, Lee JH, Yi JS, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg 2008;32:1552-8. [Crossref] [PubMed]
- Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J 2013;2:147-59. [Crossref] [PubMed]
- Jones MR, Mohamed H, Catlin J, et al. The presentation of lymph nodes in Hashimoto's thyroiditis on ultrasound. Gland Surg 2015;4:301-6. [PubMed]
- Persson HW, Hertz CH. Acoustic impedance matching of medical ultrasound transducers. Ultrasonics 1985;23:83-9. [Crossref] [PubMed]
(English Language Editor: J. Gray)


