
The effect of atrial fibrillation on perioperative outcomes in patients with pancreatic cancer undergoing open pancreaticoduodenectomy: analysis of the National Inpatient Sample
Introduction
Pancreatic cancer is one of the most deadly neoplasm in the United States and it is the fourth leading cause of death from cancer (1). Pancreaticoduodenectomy (PD) is reference surgical option for the resection of pancreatic tumors (2). During a PD, pancreatic head, common bile duct, gallbladder, and duodenum along with some part of the proximal jejunum need to be removed, and partial gastrectomy might be conducted (3). The approach of PD includes open pancreaticoduodenectomy (OPD), laparoscopic pancreaticoduodenectomy (LPD) and robot-assisted pancreaticoduodenectomy (RPD).
Atrial fibrillation (AF) is the most common arrhythmia (1–2% in the general population) (4), and it is associated with increased perioperative mortality and morbidity (5). Several studies have demonstrated that AF is related to higher mortality and morbidity, as well as more perioperative complications (6,7). However, there has been paucity of literature evaluating the impact of AF on patients with pancreatic cancer undergoing OPD, especially large-scale studies. Therefore, we carried out this study, in the hope of exploring our knowledge in this field.
We presented the following study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at https://dx.doi.org/10.21037/gs-21-116) (8).
Methods
Data source
Supported by the Healthcare Cost and Utilization Project (HCUP), the National Inpatient Sample (NIS) is one of the largest inpatient administrative databases in the U.S. It represents approximately 20% of hospitalization in the USA, and it extracts data of nearly 8 million hospital discharges from more than 1,000 hospitals per year. The diagnosis and procedure codes in NIS database are based on the International Classification of Diseases, Ninth Revision, and Clinical Modification, Ninth Revision (ICD-9-CM). The NIS contains over 100 patient level data elements (demographic, diagnostic, and procedural) as well as hospital data such as location, median income of patients by zip code, teaching status, etc. Data from 2012 to 2014 were investigated. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was exempt from institutional review board evaluation of Beijing Friendship Hospital, Capital Medical University and informed consent was not required because this study was based on the NIS database, which includes fully anonymized and de-identified data.
Study population
We searched for patient discharge data (ICD-9-CM) and hospital characteristics of patients ≥18-year-old during years 2012–2014. The population of interest in this study was patients who underwent OPD, identified by ICD-9-CM procedure code 52.7. Robotic assisted procedures (ICD-9 procedure code 17.4) and laparoscopic procedures (ICD-9 procedure code 54.21) were excluded. Non-elective procedures were also excluded. Patients were then stratified into 2 groups based on the presence of AF (ICD-9-CM code 427.3). Patient demographics (age, sex, income), hospital characteristics (location, teaching status and size) and common comorbidities were identified with ICD-9-CM codes (Table S1). Patients with missing data were excluded from our analyses.
Study endpoints
The primary endpoint of this study included (I) in-hospital mortality; (II) length of stay (LOS); (III) cost and (IV) in-hospital complications, including gastrointestinal (GI) anastomotic leakage, GI bleeding, acute kidney injury (AKI), gastroparesis, blood transfusion, cardiac complications, respiratory complications, pneumonia, pulmonary embolism (PE), urinary tract infection (UTI), cardiac arrest, postoperative sepsis, postoperative shock and wound complications. The ICD-9 codes of gastrointestinal (GI) anastomotic leakage are 44.61, 44.63, 44.69, 46.71, 46.72, 46.79 (procedure codes). Different kinds of anastomotic leakages like leakages of the pancreatic anastomosis or hepaticojejunostomy cannot be differentiate with ICD-9 codes.
Statistical analysis
We used a survey-specific method, with commands svyset and svy with pweight using DISCWT for 2012 to 2014 to generate nationwide estimates for each year. The stratum statement specifies NIS_STRATUM as the stratum identifier and the cluster statement specifies HOSP_NIS as the cluster identifier.
Descriptive statistics was used to compare demographic characteristics and outcomes between OPD patients with and without AF. The baseline characteristics were compared between the AF group and control group using the Chi-square test for categorical variables and Student’s t-test for continuous variables. In our multivariable regression model, we assessed differences in binary outcomes by using logistic regression [logistic command in Stata (StataCorp)] and in continuous outcomes by using linear regression (Stata command regress). All above were carried out in weighted samples.
We also performed propensity score matching model. In this model we were using Kernel matching with common support to general propensity weights. Patient characteristics (age, race, sex, insurance, etc.), hospital characteristics (location and size, etc.) and comorbidities (congestive heart failure, coronary artery disease and chronic kidney disease, etc.) were incorporated into our propensity score matching model. The balance was tested and satisfied between the non-AF group and AF group. Then we performed multivariate regression model using new propensity weight (binary outcomes by using logistic regression and in continuous outcomes by using linear regression).
Analyses were performed using Stata version 14 (StataCorp, College Station, TX). All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Patient and hospital characteristics
A total of 12,785 patients aged ≥18 years underwent OPD during years 2012–2014 (Figure 1). Among them, 11,469 (90%) had no AF and 1,316 (10%) had AF. There were significantly more male, and older patients in AF group. There was no statistically significant difference in median annual income, hospital bed size, region, location, and teaching status. As for comorbidities, history of myocardial infarction (MI), hypertension, diabetes mellitus, history of stroke, peripheral vascular disease (PVD), congestive heart failure (CHF), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), valvular heart disease (VHD), coronary artery disease (CAD) and cirrhosis were more prevalent in the AF group. There was no significant difference in thrombocytopenia, chronic liver disease and obesity between the AF and non-AF group (Table 1).
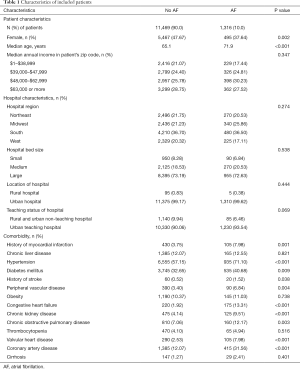
Full table
Mortality
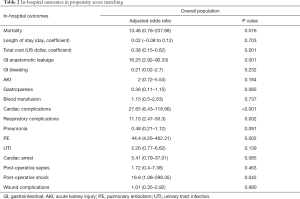
The in-hospital mortality rate for all patients was 3.17%. Mortality in AF group was higher than that in non-AF group (7.60% vs. 2.66%). Multivariable regression analysis revealed that there is a trend that AF was associated with higher mortality in patients with pancreatic cancer undergoing OPD (adjusted OR 3.57, 95% CI: 0.86–14.87, P=0.08). This was also seen in propensity score matching model (adjusted OR 13.46, 95% CI: 0.76–237.88, P=0.076) (Table 2).
Full table
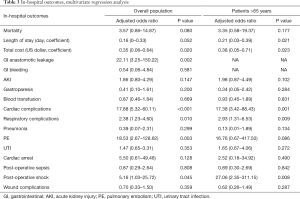
We further focused on older patients (age >65). However, results of multivariable regression analysis still showed no significant difference (adjusted OR 3.35, 95% CI: 0.58–19.37, P=0.177) (Table 3, Figure 2).
Full table
Length of stay
The in-hospital mean LOS for all patients with OPD was 12.1 days. Unadjusted analysis showed patients with AF had longer LOS (14.6 vs. 11.8 days). However, after multivariate regression we didn’t get significant result (adjusted coefficient 0.16, 95% CI: 0–0.33, P=0.052). In propensity score matching model, we did not get significant result either (adjusted coefficient 0.02, 95% CI: −0.08 to 0.12, P=0.703) (Table 2).
Interestingly, significant difference was found in the subgroup analysis in patients older than 65 years (adjusted coefficient 0.21, 95% CI: 0.03–0.39, P=0.021) (Table 3, Figure 2).
Cost of hospitalization
The in-hospital mean cost for all patients with OPD was 40,450.05 US dollars. Unadjusted analysis showed patients with AF had a significantly higher cost of hospitalization (54,601.47 vs. 38,812.80 dollars). After multivariate regression we still got significant result (adjusted coefficient 0.35, 95% CI: 0.06–0.64, P=0.02). Similar result was found in propensity score matching (adjusted coefficient 0.38, 95% CI: 0.15–0.62, P=0.001) (Table 2).
Among older patients (>65 years old), there was still significant difference between AF group and non-AF group on cost of hospitalization (adjusted coefficient 0.38, 95% CI: 0.05–0.71, P=0.023) (Table 3, Figure 2).
In-hospital complications
We also focused on in-hospital complications using univariate screening and multivariate regression. In unadjusted data, AF group has significantly higher incidence of acute kidney injury (15.97% vs. 7.24%), blood transfusion (27.38% vs. 20.27%), cardiac complications (12.55% vs. 1.18%), respiratory complications (17.49% vs. 9.98%), pneumonia (6.08% vs. 2.92%), cardiac arrest (3.42% vs. 0.87%), post-operative sepsis (10.65% vs. 5.54%) and post-operative shock (3.42% vs. 1.53%).
After multivariate regression, we got different results. Patients in AF group has significantly higher incidence of GI anastomotic leakage (OR 22.11, 95% CI: 3.25–150.22, P=0.002), cardiac complications (OR 17.88, 95% CI: 5.32–60.11, P<0.001), respiratory complications (OR 2.38, 95% CI: 1.23–4.60, P=0.01), PE (OR 18.53, 95% CI: 2.67–128.62, P=0.003), postoperative shock (OR 5.16, 95% CI: 1.03–25.72, P=0.045) than non-AF group. While no difference in GI bleeding, AKI, gastroparesis, blood transfusion, pneumonia, UTI, cardiac arrest, postoperative sepsis and wound complications was observed.
Similar result was again seen in propensity score matching model. Patients in AF group has significantly higher incidence of GI anastomotic leakage (OR 16.25, 95% CI: 2.92–90.33, P=0.001), cardiac complications (OR 27.65, 95% CI: 6.43–118.96, P<0.001), respiratory complications (OR 11.15, 95% CI: 2.47–50.3, P=0.002), PE (OR 44.4, 95% CI: 4.26–462.31, P=0.002), postoperative shock (OR 16.8, 95% CI: 1.08–288.35, P=0.042) than non-AF group. While no difference in GI bleeding, AKI, gastroparesis, blood transfusion, pneumonia, UTI, cardiac arrest, postoperative sepsis and wound complications was observed (Table 2).
Among patients older than 65 years, significantly higher incidence of cardiac complications (OR 17.38, 95% CI: 3.42–88.43, P=0.001), respiratory complications (OR 2.93, 95% CI: 1.31–6.53, P=0.009) and postoperative shock (OR 27.06, 95% CI: 2.35–311.15, P=0.008) was found in AF group (Table 3, Figure 2).
Discussion
PD is the currently the standard surgical treatment for tumors in the head of the pancreas, the lower end of the common bile duct, the duodenal papilla, and certain benign lesions. Currently the perioperative mortality of PD is less than 5% (9-12), however, the incidence of perioperative complications can be 27.1% or higher (13). Despite the advances in technology, the prognosis of PD remains poor, with 5-year survival rate being only 16–20% (14-16). Nonetheless, with upcoming new chemotherapy regimens, we might be expecting better survival outcomes in the near future (17,18). Common complications of PD include pancreatic fistula, bile leakage, GI anastomotic leakage, GI bleeding, AKI, gastroparesis, cardiac complications, respiratory complications, UTI, postoperative sepsis, postoperative shock, wound complications, etc. (19,20) Previous studies have shown that perioperative complications plays an adverse role on survival and prognosis in various tumor types including esophageal cancer (21), gastric cancer (22) and colorectal cancer (23), while this holds true in pancreatic cancer also (20).
AF is the most common type of arrhythmia (4), with a prevalence of 1–2% in the general population. Patients with AF are at a higher risk of thrombo-embolic disease and they were commonly placed on long term anticoagulation therapy (24). It has been reported that AF is associated with significantly higher occurrence of perioperative complications in various surgical operations, including urinary system surgery, liver transplantation, etc. (6,7,25).
To the best of our knowledge, this is the first large-database, population-based study investigating the impact of AF on the outcomes of pancreatic cancer patients with OPD. The reports on mortality of PD varies in various studies. Cameron et al. summarized 1,000 consecutive PD and found that only 10 postoperative deaths, for a mortality of 1% (26). Kneuertz et al. reported that the 30-day mortality after PD was 2.9% (27). Our study found that from 2012 to 2014, the average overall in-hospital mortality was 3.17%, while 7.6% and 2.66% in the AF group and non-AF group, respectively. Though no statistical significance was found (adjusted OR 3.57, 95% CI: 0.86–14.87, P=0.08), this three-fold trend might suggest potential clinical significance.
In regards to utilization of hospital resources, we found that LOS was longer in AF group in unadjusted data, but no significant difference was found after multivariate analysis [14.6 vs. 11.8 days, adjusted coefficient 0.16 (0–0.33), P=0.052]. There was a significant difference in cost between the two groups [54,601.47 vs. 38,812.80 dollars, adjusted coefficient 0.35 (0.06–0.64), P=0.02]. Adjustment of anticoagulant and a thorough evaluation of cardiac function like echocardiography and exercise tolerance test might be connected to the cost increase. What is more, higher incidence of perioperative complications such as gastrointestinal anastomotic leakage, cardiac complications, respiratory complications, pulmonary embolism, and perioperative shock in AF group might contribute to the higher expenditure.
Regarding complications after PD, Kumar et al. (28) found that pulmonary complications were the leading cause for mortality after PD. Other causes of death include bile sepsis, liver failure, myocardial infarction, pancreatic leak, etc. Narayanan et al. (29) showed that the most common cause of death after PD within 90 days is multisystem organ failure, followed by post-pancreatectomy hemorrhage, and cardiopulmonary arrest from myocardial infarction or pulmonary embolus. Our study found that the AF group had more perioperative complications than the non-AF group, including GI anastomotic leakage, cardiac complications, respiratory complications, PE and postoperative shock. Among all complications, the incidence of GI anastomotic leakage in the AF group was approximately 22 times higher than that in the non-AF group. The causality is unclear and further studies would be warranted.
The NIS database cannot determine the amount of bleeding, therefore, we tried using blood transfusion as a suboptimal measure to indirectly identify intraoperative bleeding. On the other hand, the post-operative shock was not limited to hemorrhagic, but also cardiogenic shock (possibly related to cardiac complications) and septic shock (possibly related to GI anastomotic leakage). In our result, we got significantly more incidence of post-operative shock in AF group than the non-AF group, yet no statistical difference in blood transfusion was found between the two groups. We suspected that the cause of post-operative shock in AF group is more cardiogenic and / or infectious than hemorrhagic, however this is only correlation not causality limited by the retrospective character of our study.
Botwinick et al. (30) reported that patients with AF are more likely to experience gastroparesis, but our study did not find the adverse effect of AF on gastroparesis. There were 249 patients included in Botwinick’s study, of which only 13 had atrial fibrillation, so further research may be needed to confirm the relationship between atrial fibrillation and gastroparesis. In addition, no differences were found in GI bleeding, AKI, gastroparesis, blood transfusion, pneumonia, UTI, cardiac arrest, postoperative sepsis, wound complications, etc.
With the advancement of surgical techniques, an ever increasing number of elderly pancreatic cancer patients with complex comorbidities have received PD. Several studies have shown that age has an important adverse effect on the occurrence of perioperative complications and survival after PD (31,32). Therefore, we further analyzed patients aged >65 years. No significant difference was found in mortality, while there was significant difference between the two groups in LOS and cost. As for complications, the differences in various complications between the AF group and the non-AF group in the older population are similar to the overall population. It can be seen that although age will increase complications and mortality, it will increase evenly between the two groups. Further studies are needed to reveal the influence of age on perioperative complications and death after PD.
For pancreatic cancer patients with atrial fibrillation undergoing PD, a thorough and careful evaluation of cardiac function and adjustment of the use of anticoagulants before surgery would be beneficial. Patients with atrial fibrillation often take anticoagulant drugs like warfarin, rivaroxaban or apixaban regularly. Since PD is associated with high risk for bleeding, while pancreatic cancer coupling with atrial fibrillation is associated with high risk of thromboembolism, we have to take perioperative anticoagulation plan seriously.
For patients with atrial fibrillation who take warfarin orally, omitting warfarin for 5 days before surgery and checking the INR on the day before surgery has been recommended (33,34). The use of bridging while holding oral anticoagulants has become common clinical practice, though some current evidence argue that bridging may not significantly reduce thromboembolic events, yet increasing major adverse cardiovascular events and bleeding (35,36). Douketis et al. concluded that forgoing bridging anticoagulation was noninferior to perioperative bridging in patients with atrial fibrillation (37). According to the ACC Expert Consensus, if the patient’s thrombosis risk is low (CHA2DS2-VASc Score ≤4), bridging is not recommended; if the patient’s thrombosis risk is medium-to-high (CHA2DS2-VASc Score 5 and above) or with prior stroke or TIA, clinical judgment need to be used (33). For patients who have previously taken apixaban or rivaroxaban, it is suggested that the anticoagulants should be omitted 2–3 days before surgery and bridging is not required (33,38). However, there has been limited evidence regarding bridging, specifically for pancreatic cancer patient, in the 2017 ACC periprocedural management of anticoagulation guideline. In this case, if a complicated case is encountered, multidisciplinary team approach involving cardiologists and anesthesiologists would likely be beneficial.
There are several limitations of the present study. First of all, our study is a retrospective study based on the NIS database with all inherent shortcomings. Secondly, because NIS is an inpatient database coding with ICD-9-CM, complications without ICD-9 code, such as pancreatic fistula, bile leakage, cannot be analyzed. In addition, tumor information such as tumor type, stage, and neoadjuvant chemotherapy is absent in the NIS database, which may have an impact on the outcomes of OPD. Finally, as the follow-up information is not included within the NIS database, the postoperative outcomes is not discussed in this study. Despite these limitations, this is the first population-based study that investigated the impact of AF on the outcomes of pancreatic cancer patients undergoing OPD, providing surgeons data and serving as a basis for future prospective studies.
Conclusions
AF increases the cost of pancreatic cancer patients undergoing OPD, also increases the occurrence of cardiac complications, respiratory complications, PE, and postoperative shock. Surgeons should pay special attention to patients with atrial fibrillation, and consider working together with cardiologists and anesthesiologists to jointly develop a management plan.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81172318).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-116
Peer Review File: Available at https://dx.doi.org/10.21037/gs-21-116
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-116). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was exempt from institutional review board evaluation of Beijing Friendship Hospital, Capital Medical University and informed consent was not required because this study was based on the NIS database, which includes fully anonymized and de-identified data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Fingerhut A, Vassiliu P, Dervenis C, et al. What is in a word: Pancreatoduodenectomy or pancreaticoduodenectomy? Surgery 2007;142:428-9. [Crossref] [PubMed]
- Kang CM, Lee JH. Pathophysiology after pancreaticoduodenectomy. World J Gastroenterol 2015;21:5794-804. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. Circulation 2014;130:2071-104. [Crossref] [PubMed]
- Gialdini G, Nearing K, Bhave PD, et al. Perioperative Atrial Fibrillation and the Long-term Risk of Ischemic Stroke. JAMA 2014;312:616-22. [Crossref] [PubMed]
- Ghani KR, Anson KM, Camm AJ. Atrial fibrillation and the urologist. BJU Int 2004;94:254-5. [Crossref] [PubMed]
- Bargehr J, Trejo-Gutierrez JF, Patel T, et al. Preexisting atrial fibrillation and cardiac complications after liver transplantation. Liver Transpl 2015;21:314-20. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Mohammed S, Fisher WE. Quality Metrics in Pancreatic Surgery. Surg Clin North Am 2013;93:693-709. [Crossref] [PubMed]
- Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2017;161:1221-34. [Crossref] [PubMed]
- Luu AM, Krasemann L, Fahlbusch T, et al. Facing the surgeon's nightmare: Incidence and management of postoperative pancreatic fistulas grade C after pancreaticoduodenectomy based on the updated definition of the International Study Group of Pancreatic Surgery (ISGPS). J Hepatobiliary Pancreat Sci 2020;27:171-81. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative Factors Predict Perioperative Morbidity and Mortality After Pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126-35. [Crossref] [PubMed]
- Neeman U, Lahat G, Goykhman Y, et al. Prognostic significance of pancreatic fistula and postoperative complications after pancreaticoduodenectomy in patients with pancreatic ductal adenocarcinoma. Surgeon 2020;18:24-30. [Crossref] [PubMed]
- El Nakeeb A, El Sorogy M, Ezzat H, et al. Predictors of long-term survival after pancreaticoduodenectomy for peri-ampullary adenocarcinoma: A retrospective study of 5-year survivors. Hepatobiliary Pancreat Dis Int 2018;17:443-9. [Crossref] [PubMed]
- Luu AM, Braumann C, Belyaev O, et al. Long-term survival after pancreaticoduodenectomy in patients with ductal adenocarcinoma of the pancreatic head. Hepatobiliary Pancreat Dis Int 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Romano G, Agrusa A, Galia M, et al. Whipple's pancreaticoduodenectomy: Surgical technique and perioperative clinical outcomes in a single center. Int J Surg 2015;21:S68-71. [Crossref] [PubMed]
- Karim SAM, Abdulla KS, Abdulkarim QH, et al. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int J Surg 2018;52:383-7. [Crossref] [PubMed]
- Saeki H, Tsutsumi S, Tajiri H, et al. Prognostic Significance of Postoperative Complications After Curative Resection for Patients With Esophageal Squamous Cell Carcinoma. Ann Surg 2017;265:527-33. [Crossref] [PubMed]
- Hayashi T, Yoshikawa T, Aoyama T, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer 2015;18:368-74. [Crossref] [PubMed]
- Marra F, Steffen T, Kalak N, et al. Anastomotic leakage as a risk factor for the long-term outcome after curative resection of colon cancer. Eur J Surg Oncol 2009;35:1060-4. [Crossref] [PubMed]
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol 2018;72:2027-36. [Crossref] [PubMed]
- Higuchi S, Kabeya Y, Matsushita K, et al. Incidence and complications of perioperative atrial fibrillation after non-cardiac surgery for malignancy. PLoS One 2019;14:e0216239 [Crossref] [PubMed]
- Cameron JL, Riall TS, Coleman J, et al. One Thousand Consecutive Pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [Crossref] [PubMed]
- Kneuertz PJ, Pitt HA, Bilimoria KY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012;16:1727-35. [Crossref] [PubMed]
- Kumar SV, Prasad AS. Postoperative morbidity following Whipple’s procedure for periampullary carcinoma: a retrospective study spanning 5 years. Int. J of Res in Med Sci 2019;7:4314-9.
- Narayanan S, Martin AN, Turrentine FE, et al. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res 2018;231:304-8. [Crossref] [PubMed]
- Botwinick IC, Shonkwiler RJ, Steele J, et al. Atrial fibrillation and delayed gastric emptying. PLoS One 2011;6:e25499 [Crossref] [PubMed]
- Sukharamwala P, Prashant S, Thoens J, et al. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB (Oxford) 2012;14:649-57. [Crossref] [PubMed]
- Yuan F, Essaji Y, Belley-Cote EP, et al. Postoperative complications in elderly patients following pancreaticoduodenectomy lead to increased postoperative mortality and costs. A retrospective cohort study. Int J Surg 2018;60:204-9. [Crossref] [PubMed]
- Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation. J Am Coll Cardiol 2017;69:871-98. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative Management of Antithrombotic Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e326S-50S.
- Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation 2015;131:488-94. [Crossref] [PubMed]
- Beyer-Westendorf J, Gelbricht V, Förster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J 2014;35:1888-96. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med 2015;373:823-33. [Crossref] [PubMed]
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012;120:2954-62. [Crossref] [PubMed]




