The presentation of lymph nodes in Hashimoto’s thyroiditis on ultrasound
Introduction
Hashimoto’s thyroiditis (HT) is the most common type of thyroiditis and the most common autoimmune disease, with an annual incidence worldwide of about 1 case per 1,000 persons (1,2). In the United States and other countries where there is sufficient dietary iodine, it is the most common cause of hypothyroidism (3).
The key factor in the development of autoimmune thyroiditis is the breakdown of immune tolerance. The initial production and clonal expansion of self-reactive cells occurs in the regional lymph nodes. Later, lymphoid tissue develops directly in the thyroid gland (4,5). The cells lining the thyroid follicles (thyrocytes), which are the target for these autoreactive lymphocytes, are progressively destroyed, ultimately leading to hypothyroidism (4). The inflammation is primarily mediated by circulating antibodies against thyroid tissue (6). High serum thyroid peroxidase antibody concentrations are present in 90% of patients with HT, and high serum thyroglobulin antibody concentrations are present in 20% to 50% of these patients (3,7).
HT is characterized pathologically by lymphocytic infiltration of the interstitium, mainly lymphocytes with some plasma cells and macrophages (1,3). The lymphoid tissue is distributed within and around the lobules, and often exhibits large follicles with prominent germinal centers (1,4). The inflammatory process also results in oxyphilic changes of follicular epithelial cells, parenchymal atrophy of thyroid tissue, and varying degrees of fibrosis, imparting a firm consistency to the thyroid (8).
Neck ultrasound (US) has become the most commonly used imaging tool in patients with thyroid diseases (9,10). Thyroid US of patients with HT generally reveals diffuse enlargement of the gland, a heterogenous background, and a general decrease in echogenicity. Other sonographic findings of HT often include hypervascularity and the presence of hypoechoic micronodules with an echogenic rim (11-13).
Despite the known involvement of lymphoid tissue in the pathogenesis of HT, little investigation has been done into the pattern of cervical lymphadenopathy in these patients (14). This is of particular importance given the long debate over the relationship between HT and thyroid cancer, specifically papillary carcinoma of the thyroid (PTC) (15,16). Lymphadenopathy is often considered a finding concerning for malignancy (17). However, more recent studies have found that the presence of benign, enlarged cervical lymph nodes is common in HT patients (14,18,19). It is important to determine whether the lymphadenopathy is a result of the malignancy or the thyroiditis.
This study therefore seeks to describe the cervical lymph node findings on US in patients with HT, including size, number, anatomic location and ultrasonographic characteristics. The characterization of the sonographic pattern of lymph nodes in patients with HT may assist in improving the diagnosis of HT, as well as preventing unnecessary testing of patients for malignancy based solely on cervical lymphadenopathy.
Materials and methods
This is a retrospective study of 417 patients from Tulane University Medical Center visiting from January 2013 to September 2014. Of our patients, 366 (87.77%) were female and the average age was 52.53±14.96 years. All patients underwent a preoperative comprehensive neck US and the diagnosis of HT was made based upon final surgical pathology. As the control group, 215 patients with euthyroid goiter were selected (28 males, 187 females). All ultrasounds were performed by a single surgeon who is the senior author on this paper.
For each patient included in the study, we recorded the following: age; gender; duration of disease (expressed in days after diagnosis); thyroid volume by ultrasound; number of cervical lymph nodes, cervical level (I-VI), size (short-axis diameter, long-axis diameter, short axis: long axis-ratio), and presence or absence of fatty hilum; thyroid stimulating hormone (TSH) level; and dose of Levothyroxine (if applicable).
Statistical methods included Fisher’s exact test for the categorical variables and two-tailed Student’s t-test for the continuous variables. Statistical significance was set as (α=0.05). All the analyses were carried out using SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Our population consisted of a total of 417 patients with thyroid disease. The study group included 202 patients with HT, and the control group included 215 patients with nontoxic goiter. Disease duration for documented HT averaged 861.59 days. Gender representation in the study population overall was 87.77% female (N=366).
In the group of 215 patients with nontoxic goiter females represented 86.98% (N=187), while in the HT group females represented 88.61% (N=179) for a total of 366 females out of 417 patients overall. In the HT group, mean age was 51.11±14.92 years while the study group’s mean age was 53.90±14.91 years. There was no significant difference between the control group and the HT group in terms of age or gender distribution. These measurements are summarized in Table 1.

Full table
As expected, TSH levels were higher in the HT group (10.85±30.91 mIU/L HT group vs. 1.44±1.18 mIU/L controls, P<0.001). Mean right thyroid lobe volume as measured on ultrasound was 29.96±36.72 milliliters (mL) in controls and 21.20±27.34 mL in the HT group (P=0.08). Mean left thyroid lobe volume as measured on ultrasound was 24.53±36.22 mL in controls and 17.09±23.17 mL in the study group. The left lobe volume as measured by ultrasound was significantly larger in the control group as compared to the study group (24.53±36.22 vs. 17.09±23.17 mL, P=0.034).
The average number of total enlarged nodes per patient identified on ultrasound in the control group was significantly lower than the HT population, 0.76±1.36 vs. 2.00±2.35 nodes (P<0.001). The mean number of nodes was significantly higher in Levels I, III, and IV: 0.09±0.32 nodes in the control group for Level I vs. 0.33±0.79 nodes in the HT group (P<0.001), at Level III 0.22±0.50 nodes in the control group vs. 0.63 nodes ±1.08 in the HT group (P<0.001), and at Level IV 0.06±0.25 nodes in the control group vs. 0.19±0.44 nodes in the HT group (P=0.044). The mean number of nodes at Levels II, V, and VI was not significantly different between the two groups. The mean number of nodes at Level II was 0.21±0.67 in the controls and 0.34±0.72 in the HT group; at Level V it was 0.04±0.21 in the controls and 0.08±0.32 in the HT group, and at Level VI it was 0.02±0.18 in the controls and 0.03±0.27 in the HT group.
Measurements in the Level IV compartment revealed a short-to-long axis ratio of 0.32±0.05 in the control group and 0.52±0.19 in the HT group, (P<0.001). All other lymph node measurements are listed in Table 2.
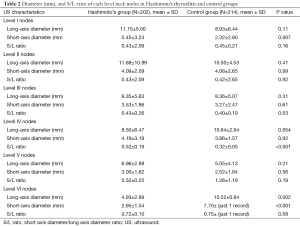
Full table
Node status was characterized as benign appearing in 92.91% of the control group (N=131) and 94.44% (N=306) of the HT group. Node status was characterized as suspicious for malignancy by ultrasonographic features in 7.09% (N=10) of the control group and 5.56% (N=18) of the HT group (P=0.53). Fatty hilum was identified on 3.36±2.00 lymph nodes per HT subject and 2.11±1.43 lymph nodes per goiter subject (P<0.001). These measurements are summarized in Table 3.

Full table
Discussion
Evaluation of lymph nodes is a critical component in the diagnosis of patients with thyroid pathology, particularly considering the frequency of co-existing benign and malignant disease and the propensity of malignant thyroid disease to metastasize to regional lymph nodes. Imaging modalities used to evaluate lymph nodes in the setting of thyroid disease must therefore have accurate, validated radiologic criteria for the differentiation of benign and malignant lymph nodes.
In this study, the aim was to evaluate size and number of cervical lymph nodes in HT to establish an accurate radiologic threshold. Lymph node size criteria for biopsy are commonly used for many malignancies of the head and neck, including thyroid cancers, although there is conflicting data regarding their accuracy (20,21). Because reactive lymph nodes and those involved in malignancy can be equal in size, importance must be placed on appropriately interpreting sonographic findings in context (22).
The association between many autoimmune disorders and enlarged lymph nodes demands a similar investigation into HT. The ultrasonographic patterns of lymphadenopathy in autoimmune thyroiditis may allow for the creation of size-driven cutoffs. Indeed, HT has been linked to an increased presence of benign hyperplastic lymphadenopathy (18,23). Our results are similar to a study that identified those paratracheal lymph nodes more often in patients with chronic autoimmune thyroiditis versus controls. Importantly, regional lymph nodes are known to be involved in early disease; activation of T cells in thyroid draining nodes precedes the clonal expansion of autoreactive T and B cells, while subsequently, lymphoid tissue often develops in the gland itself (5,24).
The benign lymphadenopathy common to autoimmune thyroiditis may mimic pathologies such as malignancy (25). Unnecessary and invasive interrogations of lymph nodes are often performed solely based on these characteristic histologic features of T-zone dysplasia with hyperplastic follicles, (1,26). In addition to size criteria for biopsy, suspicion for malignancy based on other morphologic features would likely increase the specificity of sonographic studies, reduce false positive results, and decrease unnecessary invasive procedures (20,27).
Previous studies of PTC metastases demonstrated frequent involvement of Levels II, III, and IV lymph node segments (28). This suggests that these lymph nodes may directly drain the gland, and would be the most likely to be involved in other benign thyroid pathology (29). In our study, lymph nodes in Levels III and IV showed a statistically significant increase in number identified over the control group. This is consistent with the proposed pattern of lymph nodal drainage seen in inflammatory thyroid disease. However, no significant difference in size of short- or long-axis was detected at any level of lymph node between the HT and control groups.
Biopsy of enlarged cervical lymph nodes based solely on size as a marker for potential malignancy is myopic and outdated; size is only one parameter that may be useful in the evaluation for thyroid cancer. Current accepted practice involves surveillance for well-differentiated thyroid malignancy, and observance for lymph nodes sized 5-8 mm with benign features on imaging (29). Benign lymph node features, such as fatty or echogenic hilum, may help guide further evaluation. These features are usually present in the setting of inflammation and help to delineate malignant versus benign processes (30). In our study, Level IV demonstrated a large increase in the S/L ratio (P≤0.001) between the control and HT groups: 0.32±0.05 in the controls and 0.52±0.19 in the HT group. The clinician should note that, although this data is statistically significant, a difference of only 0.2 cm may be difficult to discern in the clinical setting.
Our data demonstrated no significant difference in S/L ratio between patients with HT and goiter at all other levels (20). Our knowledge of the pathogenesis of HT calls into question standard size-based cutoffs for biopsy, and a recent study supported this conclusion. In this study we showed that HT seems to be associated with an increased number of enlarged cervical lymph nodes, particularly in levels III, and IV. However, our data do not suggest any difference in size of lymph nodes at any level between patients with inflammatory versus non-inflammatory thyroid pathology.
A limitation of this study is that patients in the HT group were examined during different stages of the disease process. While all patients’ diagnoses were confirmed on surgical pathology, not all had been treated with levothyroxine. As described above, the thyroid gland and lymph nodes in these patients undergoes inflammatory changes secondary to prolonged attack by the host immune system. It is less certain, however, if the levothyroxine therapy would lessen the inflammatory changes in the lymph nodes. It is also uncertain if the lymph nodes mimic the same fluctuations in size and inflammation as the thyroid gland throughout the course of the disease (31). To that end, it would be of benefit to have larger studies that stratify patients based on TSH level, a known marker of inflammatory status.
Conclusions
HT patients present with an increased number of enlarged cervical lymph nodes, seen mainly in levels III and IV. However, there is no significant increase in lymph node size or suspicious features.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev 2014;13:391-7. [PubMed]
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55-68. [PubMed]
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55. [PubMed]
- Ahmed AM, Ahmed NH. History of disorders of thyroid dysfunction. East Mediterr Health J 2005;11:459-69. [PubMed]
- Chistiakov DA. Immunogenetics of Hashimoto’s thyroiditis. J Autoimmune Dis 2005;2:1. [PubMed]
- Armengol MP, Juan M, Lucas-Martín A, et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol 2001;159:861-73. [PubMed]
- Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician 2000;61:1047-52, 1054. [PubMed]
- Amani HK. Histopathologic and immunohistochemical features of Hashimoto thyroiditis. Indian J Pathol Microbiol 2011;54:464-71. [PubMed]
- Pedersen OM, Aardal NP, Larssen TB, et al. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 2000;10:251-9. [PubMed]
- Lee JH, Anzai Y. Imaging of thyroid and parathyroid glands. Semin Roentgenol 2013;48:87-104. [PubMed]
- Butch RJ, Simeone JF, Mueller PR. Thyroid and parathyroid ultrasonography. Radiol Clin North Am 1985;23:57-71. [PubMed]
- Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med 1996;15:813-9. [PubMed]
- Anderson L, Middleton WD, Teefey SA, et al. Hashimoto thyroiditis: Part 1, sonographic analysis of the nodular form of Hashimoto thyroiditis. AJR Am J Roentgenol 2010;195:208-15. [PubMed]
- Sahlmann CO, Meller J, Siggelkow H, et al. Patients with autoimmune thyroiditis. Prevalence of benign lymphadenopathy. Nuklearmedizin 2012;51:223-7. [PubMed]
- Cunha LL, Ferreira RC, Marcello MA, et al. Clinical and pathological implications of concurrent autoimmune thyroid disorders and papillary thyroid cancer. J Thyroid Res 2011;2011:387062.
- Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 2013;98:474-82. [PubMed]
- Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab 2013;98:3131-8. [PubMed]
- Serres-Créixams X, Castells-Fusté I, Pruna-Comella X, et al. Paratracheal lymph nodes: a new sonographic finding in autoimmune thyroiditis. J Clin Ultrasound 2008;36:418-21. [PubMed]
- Paksoy N, Yazal K. Cervical lymphadenopathy associated with Hashimoto’s thyroiditis: an analysis of 22 cases by fine needle aspiration cytology. Acta Cytol 2009;53:491-6. [PubMed]
- Steinkamp HJ, Cornehl M, Hosten N, et al. Cervical lymphadenopathy: ratio of long- to short-axis diameter as a predictor of malignancy. Br J Radiol 1995;68:266-70. [PubMed]
- van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol 1998;19:695-700. [PubMed]
- Chan JM, Shin LK, Jeffrey RB. Ultrasonography of abnormal neck lymph nodes. Ultrasound Q 2007;23:47-54. [PubMed]
- Brancato D, Citarrella R, Richiusa P, et al. Neck lymph nodes in chronic autoimmune thyroiditis: the sonographic pattern. Thyroid 2013;23:173-7. [PubMed]
- Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol 2012;19:181-6. [PubMed]
- Patel BN, Kamaya A, Desser TS. Pitfalls in sonographic evaluation of thyroid abnormalities. Semin Ultrasound CT MR 2013;34:226-35. [PubMed]
- Kojima M, Nakamura S, Oyama T, et al. Autoimmune disease-associated lymphadenopathy with histological appearance of T-zone dysplasia with hyperplastic follicles. A clinicopathological analysis of nine cases. Pathol Res Pract 2001;197:237-44. [PubMed]
- Lo CP, Chen CY, Chin SC, et al. Detection of suspicious malignant cervical lymph nodes of unknown origin: diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy with nodal size and central necrosis correlate. Can Assoc Radiol J 2007;58:286-91. [PubMed]
- Eskander A, Merdad M, Freeman JL, et al. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: a systematic review and meta-analysis. Thyroid 2013;23:583-92. [PubMed]
- Stack BC Jr, Ferris RL, Goldenberg D, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 2012;22:501-8. [PubMed]
- Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol 2005;184:1691-9. [PubMed]
- Korzeniowska K, Jarosz-Chobot P, Szypowska A, et al. L-thyroxine stabilizes autoimmune inflammatory process in euthyroid nongoitrous children with Hashimoto’s thyroiditis and type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol 2013;5:240-4. [PubMed]


