Remote access thyroid surgery
Introduction
The adoption of remote access thyroid surgery; especially robot-assisted thyroidectomy, has gained popularity in recent times. Advancement in the use of ultrasound (US) and Doppler US for screening thyroid pathologies have resulted in an increased detection of thyroid nodules as well as thyroid cancer consequently increasing the number of thyroid surgeries performed. Conventionally, open thyroidectomy has been the treatment of choice, however, the visible neck scar is undesirable for many patients. With technical advancement, these procedures transitioned from conventional to video-assisted thyroidectomy and, lately, to robot-assisted approach for better cosmesis. Video-assisted endoscopic thyroidectomies were first pioneered by Miccolli et al. (1). Shortly after, Bellantone et al. reported the safety and feasibility of performing central and lateral lymph node dissections endoscopically (1,2). There have been several reports of remote access techniques for thyroidectomy utilizing surgical ports in several locations outside the neck including the anterior chest wall, post auricular-occipital area, axillary region and sub-clavicular region. Although it requires patience and a particularly sophisticated skill set, the axillary approach has been proved to be among the most feasible approaches. To facilitate the ease of remote access thyroid surgery, the application of robotic technology in thyroid surgery emerged to overcome some of the technical challenges associated with endoscopic remote-access thyroid surgery.
Since then many surgeons have advanced their experiences to robotic thyroid surgery in their current practice. This transition marked a hike by 30% from 2010 to 2011 (3). The two most common approaches that are currently seen in practice: (I) robotic-assisted trans-axillary; and (II) retro-auricular thyroidectomy using the Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA). It was in earlier 2000’s that the use of gasless endoscopic thyroidectomy was practiced using a transaxillary incision (4). However, the heavier robotic arms of the older Da Vinci robotic system made it difficult to utilize it in the deep and narrow working space (4).
Different minimally invasive approaches to thyroid surgery
We will group these different remote access approaches into two: (I) the cervical/direct approaches; and (II) the extra-cervical/remote access approaches.
The cervical/direct approaches
These approaches involve placing a small incision in the anterior or lateral neck. Blunt dissection is used to create the operating space, and it is maintained either with low pressure CO2 insufflation or by external skin retraction. Such approaches include:
Video-assisted central approach, gasless or MIVAT (minimally invasive video-assisted thyroidectomy)
A central incision above the sternal notch that is approximately one inch in size is made, which provides direct access to the thyroid bilaterally. It is highly recommended to have a two assisting surgeons: one to retract, and the other to hold the endoscope. The strap muscles are identified and separated at the midline and then elevated off the anterior and lateral surface of the thyroid gland with standard optics. Small retractors are then utilized to retract the strap muscles laterally and the upper pole of the thyroid gland medially. A 5 mm angled endoscope is then inserted directly into the incision. Blunt dissection of the pertinent cervical anatomy is performed under video-assisted control. Gentle dissection of the superior thyroid vascular pedicle is initiated along the long axis of these vessels. To free the medial aspect of the superior pole of the thyroid gland, the harmonic scalpel is used. If the pyramidal lobe is present, it’s freed via lateral approach first then superiorly from its infra-hyoid tract. The inferior and lateral aspects of the thyroid gland are then mobilized and the middle thyroid vein is transected. The inferior parathyroid gland is then visualized and dissected laterally and maintained on its vascular supply. The thyroid gland is then extracted through the cervical incision and the isthmus and the ligament of berry are divided.
The main advantage of this approach is the direct access and small neck incision. There have been multiple published articles reporting less operative pain, better cosmesis, shorter hospital stay and the ability to perform these surgeries on outpatient basis (1,5). Furthermore, some surgeon would argue the safety and feasibility of such approach in patients with a nodule size >3 cm in diameter and >30 mL in volume (6).
Lateral endoscopic approach
A plane between the SCM and the carotid sheath laterally and the strap muscles medially is used. It needs to be mentioned that this approach is best used for unilateral lesions and revision cases.
Three to four ports are utilized during this procedure, a 10 mm optic port and two to three operating ports. The operating port is placed on the medial border of the sternocleidomastoid (SCM) muscle on the side of the lesion. This approach allows a direct access to the posterior aspect of the thyroid lobe. This eliminates the need for dissection of the strap muscles. CO2 insufflation (~8 mmHg) is used to maintain the working space. The superior vascular pedicle is then divided with the harmonic scalpel and the recurrent laryngeal nerve (RLN) is identified and traced along its entire length. The superior and inferior parathyroid glands are identified and preserved and the inferior thyroid vessels are then divided. The ligament of berry is then divided and the specimen is extracted through the 1.5 cm incision for division of the isthmus.
Anterior endoscopic approach
This approach permits a bilateral dissection of the thyroid gland due to its utilization of a midline access. Four trocar sites are site are used for this approach. The first is for a 5 mm optical trocar, which is inserted just above the suprasternal notch. Two sites are used for 2 mm trocars each, and the fourth site is used for a 5 mm trocar all of which are placed at the superior medial border of the SCM muscle. All vital structures are identified and dissected using ultrasonic shears. The thyroid gland is then extracted through the superolateral trocar.
The extra-cervical/remote access approaches
These approaches require placing incisions outside the neck, requiring extensive dissection under the skin. The operating space is then maintained by either CO2 insufflation or external retraction by specially designed skin retractors. Recently, the application of robotic technology to further assist the surgeon in accomplishing these techniques facilitated remote access thyroid surgery and helped avoid the need for insufflation. There are several advantages of robotic surgery that overlay endoscopic approach such as its high definition 3-dimensional camera system, greater freedom of motion, and multi-articulated tremor free endoscopic arms that facilitates surgeons to perform easier in a restricted narrow space favoring surgical completeness. Thus, the safety and efficacy of these approaches allow many head and neck surgeons to remove the thyroid gland with highly improved cosmetic outcomes (7-9). Despite its successful outcomes in resection of thyroid lesions, its financial burden and associated post-operative complications preclude its use by many surgeons.
The surgical robot is designed in such a way that allows the surgeon to facilitate retraction, surgical field vision, and provides two arms to operate, while still maintaining traction and counter-traction. The three robotic instruments (Maryland dissector, ProGrasp forceps and Harmonic curved shears) are utilized to orient thyroid tissue using a dual channeled camera system. The camera is placed through the axillary/retro-auricular incision using an endoscope with a 30-degree down orientation. Electrocautery, a vascular DeBakey forceps and various retractors (army-navy, right-angled and lighted breast retractors) are used to create and elevate a subcutaneous flap (Tables 1,2). This leads to a greater working space that allows the surgeon to operate with a superior field vision. Though the learning curve for novice surgeons showed excellent results, however, it is still essential to have a significant experience for this approach (10). Literature reports a significant reduction in console time after 40-50 cases (11,12).

Full table

Full table
Absence of a visible neck scar attracts patients, especially females, to opt for remote access procedures. However, it is essential to carefully select patients and adequate work up with physical examinations and screening imaging procedures are necessary when considering remote access approaches. Thyroid nodules should be properly assessed for size, location, laterality, and presence of metastatic lymph nodes. Patient’s preferences in order to achieve favorable scar should be properly addressed. The location, length, design and healing assess the overall quality of the surgery. Table 3 outlines various guidelines that are necessary for its safe implementations (Table 3). Ideal candidates for robot-assisted thyroidectomy are (I) small or average body habitus (body mass index <30 kg/m2) young patients, with history of keloid or hypertrophic scar formation or do not desire a visible neck scar. It is crucial to conserve the selection criteria, primarily, during the beginning of the surgeon’s learning curve. However, our group has reported successful outcomes for the trans-axillary approach with 60% of our patients being overweight or obese with a thyroid nodule size of 2.5 cm (7).

Full table
Trans-axillary approach
Patient positioning
Patient is placed supine under general anesthesia with arm and shoulder placed at the same vertical height. The neck is slightly extended. Adequate padding of the forearm and elbow is required to prevent nerve stretch. Intubation with a NIM endotracheal tube (Medtronic Xomed, Jacksonville, FL, USA) is used to allow intraoperative RLN monitoring (Figure 1). The arm of the lesion side is placed in a cephalad position and flexed above the head (Modified Ikeda’s arm position). In the case of a total thyroidectomy, arm ipsilateral to the larger lobe of the thyroid is positioned for incision. One should note that patients with disabled cervical or shoulder range of motion are not ideal candidate for this approach. Additionally, median and ulnar nerves (Figure 2) are routinely monitored using somatosensory evoked potentials (SSEP) (Biotronic, Ann Arbor, MI, USA). The SSEP had not been universally adopted by surgeons performing these approaches. Instead, the ipsilateral arm is careful positioned and fixed on an arm board to manage shortest distance from axilla to thyroid bed. However, this, in our opinion results in increasing the distance to dissect the thyroid bed.
Chung and colleagues described another arm position by rotating the ipsilateral arm to the lesion to 180 degrees cephalad, padding and then placing it on the board. However, this was not very well accepted by many patients in western population. It is a general practice by many robotic surgeons to perform an intraoperative US examination prior to skin incision. This aids in localizing the thyroid lesion and examine the relationship of the surrounding structures to the thyroid gland in the dissecting plane.
Skin incision
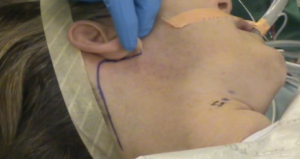
A transverse line is drawn between the sternal notch and axilla to mark the inferior limit of the incision that is directed posteriorly to ensure the incision will be hidden. Figure 3 displays a well-hidden incision in the axillary fold (Figure 3). A 60-degree oblique line is drawn from the thyrohyoid membrane to the axilla (Figure 4) to determine the superior limit of the incision. 10 mL of 1% lidocaine with 1 in 200,000 adrenaline is infiltrated. A 5-6 cm vertical incision is made intersecting the oblique line and the anterior axillary line as the superior limit. The intersection of the transverse line with the anterior axillary line defines its inferior limit. Every attempt should be made to reduce cicatrix hypertrophy by proper handling of the skin. It is recommended to use a breast fold trocar for an easier operative technique for surgeons in learning process. A newer approach of robotic procedure requires two-incision technique. In addition to above, a single anterior chest 0.6-0.8 cm skin incision in the medial fold of the breast on the lesion side is made (13). A trocar is then used with one of the robotic arms docked to the cannula that assists in the manipulation, retraction and dissection of the thyroid gland.
In the setting of performing the procedure with CO2 insufflation, three 5 mm incisions are placed below the anterior axillary line equidistant apart or one 30 mm incision is made for a 12 and 5 mm trocar, apart from the third trocar (5 mm). A modification of this approach has also been described using a second incision inferior to the axillary incision for the placement of the fourth arm for retraction. This inferior based access port can later be used as a site for surgical drain placement. This modification allows easier placement of a fourth arm while not creating an visible anterior chest wall incision; especially relevant for surgeons transitioning from a two incision to single incision trans-axillary approach (14).
Creating a working space
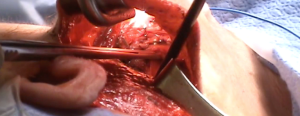
Using the monopolar electrocautery, a subcutaneous flap is prepared for dissection to create the subplatysmal plane anterior to the pectoralis fascia up to the clavicle. A wound protector (Alexis wound retractor system from Applied Medical, CA, USA) is used to protect the axillary wound edges from burns of electrocautery or the harmonic scalpel. Retractors are used to maintain a direct vision of dissection area using extended-tip bovie for deeper sections. Following clavicle identification, the 2-heads of the SCM muscle are dissected through (Figure 5) creating a wide access to the midline of the neck through the axilla. This could be challenging in the patients with large fad pad around neck such as in obese population. The assistant is, then, instructed to pull the skin away from the tunnel so as to avoid development of “buttonholes” into the skin. When using the lightened skin retractor, dissection should be carried out deep and lateral to the retractor, thus, minimizing risk of skin injury. The working space is defined by the clavicular head to just above the omohyoid muscle that correlates with the superior pole of the thyroid lobe. The sternothyroid muscle is approached through the small window between the sternal head of the SCM (medially) and the clavicular head of the SCM (laterally). The thyroid lobe is located under the strenothyroid muscle. Dissection of the uppermost fibers of sternothyroid muscle is carried out to reach the superior pole of the gland. The harmonic scalpel then creates an adequate space between the two heads of the SCM. Using Chung retractor the strap muscles are lifted anteriorly to create a working space exposing the anterior surface of the thyroid gland (Figure 6). After the Chung retractor is placed, the anesthesiologist should confirm adequate padding of the neck and shoulders. According to surgeon’s preference, the two-incision technique is opted at this point. It should be noted that the placement of the chest wall trocar after the retractor apparatus confirms proper positioning of the chest wall retractor below the Chung retractor. Conversely, it would prevent the entry of the chest wall retractor under the Chung retractor.
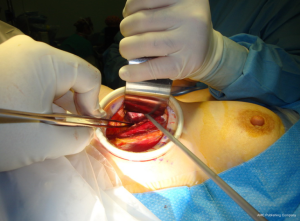
For CO2 insufflation, a flexible laparoscope with carbon dioxide insufflation at 4-9 mmHg pressure is introduced before starting sharp dissection to dissect an avascular plane between the platysma and the pectoralis major muscle. Next the plane between the SCM muscle and the sternohyoid muscle is developed to elevate the steronothyroid muscle and allow retraction anteriorly, exposing the ipsilateral thyroid gland.
Docking stage
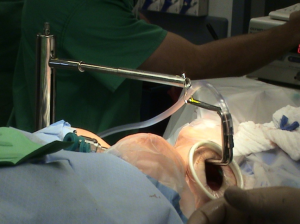
After insertion of robotic arms through axillary skin incisions, the Da Vinci model Si robot (Intuitive, Sunnyvale, CA, USA) is docked at the contralateral side of the operative field. Three robotic arms, the 30-degree down looking endoscope, the harmonic scalpel and the Maryland forceps, are inserted through the axillary incision. The Harmonic curved shears are placed in the robotic arm that corresponds to the surgeons’ dominant arm. Because harmonic scalpel does not provide freedom of motion, it can also be moved between the other robotic arms to improve visualization. Recent introduction of a vessel sealer, larger than the harmonic scalpel, with wristed movements has been utilized. It is necessary to maintain a distance between Maryland dissector and the Harmonic scalpel to avoid risk of possible collision between the two robotic arms. The 30 degrees endoscope is then place in a downward view angle onto the thyroid bed.
Surgical resection of thyroid gland
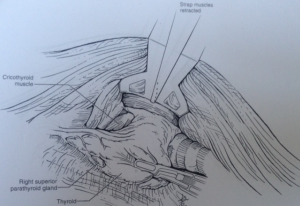
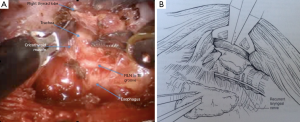
Retraction of thyroid gland is carried out using the ProGrasp retractor (Figure 7). The middle thyroid vein is, then, divided using the Harmonic scalpel. The superior pole of the thyroid gland is pulled into medio-inferior direction using the ProGrasp forceps, and the superior thyroid vessels are ligated individually using the Harmonic curved shears. Special attention should be paid to ligate all the vessels close to the thyroid gland to avoid any injury to the external branch of the superior laryngeal nerve. The thyroid gland is carefully and gradually dissected from the cricopharyngeal and cricothyroid muscles (CTMs). Dissection of the superior pole is continued until the superior parathyroid gland is exposed and released. Dissection of RLN near the inferior thyroid pedicle in tracheoesophageal groove is then carried out to reduce any risk of injury to anatomical structure. Traction in the tracheoesophageal groove can facilitate easy identification of the RLN, cranially. To assure functional integrity of the nerve, it is recommended to perform intraoperative nerve stimulation using RLN stimulator (Nerveana, Ventura, CA, USA) that is introduced through the incision (Figure 8).
Hemostasis is achieved by Harmonic scalpel by sealing the branches of inferior thyroid artery. However, it is extremely important to allow 3-5 seconds to elapse before the instrument gets activated as it may lead to burn injury due to its increased temperature (80 to 100 °C) during activation. After dissection of the gland, the isthmus is then divided using the Harmonic scalpel, and the resected thyroid lobe is removed through the incision. For patients being operated for carcinoma, the resected lobe is removed in an endo-catch bag to avoid tumor spillage. Closure is facilitated in a standard fashion with the placement of a surgical drain.
Robotic retro-auricular thyroid surgery
The retro-auricular approach is advantageous over the trans-axillary approach in several ways such as (I) easier positioning of the patient; (II) shorter distance to the thyroid gland, hence, less dissection leading to faster wound healing (15); (III) eliminating risk of brachial plexus paralysis; and (IV) easier flap elevation in obese patients (4). Walvekar et al. first described the feasibility of retro-auricular thyroidectomy approach with endoscopic assistance in a cadaveric model in 2010 (16). The authors mentioned the value of incorporation of robotic technology to be of advantage with this approach. Terris et al. first reported the clinical feasibility and application of robotic technology to a retro auricular approach and described as well as reported early experiences with robotic facelift thyroidectomy in 2011 (17). Terris and Singer favored a superior to inferior approach to the thyroid gland over a series of trans-axillary methods (18). Using an insufflation technique, this technique has been combined with the axillary approach to access the thyroid space (19). Interestingly, Terris’s hybrid technique, has made it possible to integrate the facelift parathyroidectomy incision (19), the Chung gasless robotic thyroidectomy (20), with the implementation of intra-operative laryngeal nerve monitoring.
Patient positioning
Under general anesthesia, the patient is placed on the operating room table in supine position. Intubation is carried out using a NIM endotracheal tube size 6.0 (Medtronic Xomed, Jacksonville, FL, USA) to allow intraoperative RLN monitoring.
Skin preparation and incision
The post-auricular area is cleanly shaved to mark the planned incision lines into the hair-bearing skin. The incision then is placed into post-auricular crease extending to the occipital hairline. The incision is descended 1 cm at a position that is obscured by ear and hair at rest completely (Figure 9).
Creating a working space
A subcutaneous flap superficial to the platysma is created using a Metzenbaum scissor and preserving the greater auricular nerve (Figure 10). Dissection is continued in the plane superficial to the platysma is till the head of the SCM muscle. The window between the two heads (sternal and clavicular heads) of the SCM muscle is identified and a working space is created using harmonic scalpel (Ethicon, Somerville, NJ). The strap muscles are identified and reflected medially to clearly expose the upper pole of thyroid gland. A modified Chung retractor (Marina Medical, Sunrise, FL, USA) is used to secure the operative pocket by placed it under the sternal head of the SCM muscle while continuing the dissection of strap muscles to allow exposure of the surgical field, and expanding the access to the parathyroid gland (Figure 11). It takes approximately 30 minutes to create a subcutaneous flap.
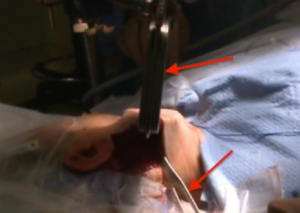
Docking stage
The Da Vinci Si system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) is docked at the contralateral side. The robotic arms are inserted through the incision (Figure 12). The endoscope is positioned centrally, a Maryland grasper is placed in the non-dominant hand, and the Harmonic is placed in the dominant hand. At this time, nerve dissection is performed at the cricothyroid membrane entrance, and integrity is confirmed by stimulation of the nerve just proximal to the inferior margins of the inferior constrictor muscle. It takes seven minutes to dock the robot. Dissection of the thyroid gland is performed as described above in the transaxillary approach.
Breast approach
This approach allows for bilateral dissection of both thyroid lobes. A trocar size range from 10-15 mm ports are inserted on both of the upper circumareolar areas of the breast. CO2 insufflation up to a pressure of 6 mmHg is used to establish the working space. This technique has two different approaches:
- The axilo-bilateral breast approach (ABBA), which utilizes a third port inserted in the axilla, which allows for specimen extraction. A 5 mm harmonic scalpel is inserted in the left breast, while an optical trocar is inserted in the right breast. To achieve good exposure, the ipsilateral strap muscles are divided. The gland is then removed through the axillary incision;
- A bilateral axillo-breast approach (BABA) has also been developed to obtain optimal visualization of both lobes. With insertion of the third and the fourth port in the left and right axilla, a total thyroidectomy is possible with this approach. With introduction of the da Vinci surgical system, BABA endoscopic thyroidectomy was combined with robotic thyroid surgery in 2008. This allowed better visualization of critical structures such as the parathyroid glands, RLNs and superior and inferior thyroidal vessels.
Chest wall approach
It’s also known as the subclavicular approach. This approach has been developed and commonly used in Asia. It’s a favored approach for bilateral thyroid resection. A 30 mm skin incision is made below the inferior border of the clavicle ipsilateral to the lesion or the larger lobe is made. Next is retracting the myocutaneous flap above the pectoralis major muscle. A blunt instrument or an endoscopic dissector is used for the initial blunt dissection. A 12 mm trocar is introduced for a flexible laparoscope. To maintain the working space, CO2 insufflation to a pressure of 4 mmHg is performed or the use of specially designed lifting devices. Two additional trocars are introduced, one 5 mm trocar is inserted inferior to the sternal notch and the other 5 mm is inserted below the ipsilateral clavicle. The strap muscles are then divided to improve exposure and dissection of the thyroid is performed with the use of ultrasonic shears. The thyroid gland is then extracted through a 30 mm incision, leaving no scar in the neck.
Experimental transoral video-assisted or robotic approaches
A German group reported transoral video-assisted thyroidectomy in a small series. This approach utilizes a 10 mm sublingual sagittal incision. Dissection through the floor of the mouth musculature to the subplatysmal plane is performed. Then carbon dioxide insufflation, followed by bilateral 10 mm vestibular incisions lateral to each mandibular canine is made. We have recently reported the addition of robotic technology to this approach in human cadavers. However, this is still considered as an experimental technique with a significantly reported conversion rate and complications. Robotic transoral periosteal thyroidectomy (TOPOT) is another trans-oral technique described in cadavers that uses sub-periosteal port placement with robot assistance to facilitate a midline access for thyroidectomy (21).
Potential complications and their management
Various concerns have arisen regarding post-operative outcomes and complications associated with robotic procedure. Therefore, it becomes crucial to adequately assess these new technologies before integrating into standard practice. At rare instances, there may arise a possibility of conversion to an open procedure, thus, should always be discussed with patients prior to undergoing surgery. Thyroidectomy via every approach accounts for the following complications:
Hypo-parathyroidism
Due to inadvertent injury to parathyroid glands, transient hypocalcemia is observed in 5-50% while permanent hypocalcemia (hypocalcemia >6 months) between 0.5-2%. Careful identification of parathyroid glands should be carried out in order to prevent ischemic injury of the glands. Patients should be warned about the presenting symptoms of hypocalcemia such as numbness, tingling, carpopedal spasm, seizures and changes in Electrocardiogram (EKG). Post-operative supplementation of calcium (oral/intravenous) is the mainstay of treatment in these patients. The dosage of oral calcium supplementation is adjusted according to serum calcium levels. If hypocalcemia persists despite receiving 2 grams of oral calcium, additional dose of 0.25-1 mcg/day calcitriol supplementation should be considered. Patients with severe hypocalcemic symptoms or refractory to oral supplementation require intravenous calcium supplementation. The American Thyroid Association guidelines on outpatient thyroidectomy require patients to be discharged with calcium, vitamin D and calcitriol supplementation if necessary (22).
Injury to the RLN
The transient and permanent injury to RLN has been reported by various studies. The rate of transient RLN palsy be 3-8% while permanent palsies (lasting more than 12 months) between 0.3-3% of cases (23). Post-operatively, patients are followed up for development of symptoms such as hoarseness, aspiration, and dysphagia. However, bilateral cord paralysis present with respiratory distress followed by immediate airway obstruction.
The patients referred to our institution are screened with a pre-and a post-operative vocal cord evaluation using a flexible laryngoscopy. This confirms the position, mobility, and functionality of the vocal cords. Patients with suspected bilateral vocal cord paralysis might require an urgent airway management with the possibility of definitive management with a tracheostomy. Therefore, utmost care is taken to prevent any intra-op injury to RLN. A standard practice to integrate visual identification and use Intraoperative Nerve Monitoring is recommended for all thyroidectomy cases.
Injury to the superior laryngeal nerve
The injury to the two branches of the superior laryngeal nerve, the external and internal branches, can go unnoticed during these surgeries. Therefore, identification and safety of these nerves are of paramount importance. The external branch innervates the CTM and the internal branch provides sensory innervation to the larynx. It has been reported that injury to these nerves are encountered in 58% of the cases (24). Symptoms such as hoarseness, vocal fatigue, and loss of high-pitched sounds may be present in the patients. Videostroboscopy and laryngeal electromyography (EMG) evaluate the affected vocal cord, which is seen to be lower than the normal cord on examination. Therefore, it is essential for surgeons to incorporate intraoperative nerve monitoring.
Neck hematoma
Approximately 1% of patients suffer neck hematoma. Though life-threatening, this can be prevented by achieving adequate hemostasis (24). Patients with suspected hematoma present with asphyxiation, airway compromise leading to re-exploration of the neck. In rare cases, immediate evacuation may be required. Surgeons are recommended to perform valsalva maneuver to minimize the risk of bleeding before closure of incision. A drain should be placed post-operatively to further reduce chances of imminent hematoma.
Infection
As any surgical procedure, the risk of infection cannot be neglected. However, there has been a significant reduction (1-2%) in reported cases of sepsis due to the advanced technology and sterilized instruments (25). Presenting signs of wound infection such as fever, cellulitis with warmth, erythema, and tenderness around incision site, superficial abscess, and leukocytosis should be looked for. Once abscess develops, these need to be drained and aspirated to send for culture. Broad-spectrum antibiotics (e.g., clindamycin, cefuroxime, ampicillin-sulbactam) are started until definitive culture results are available. Patients presenting with superficial cellulitis require antibiotic coverage for gram-positive organisms (e.g., streptococci and staphylococci). CT-guided imaging is considered preeminent for evaluation of deep abscesses in the neck. It is essential to follow the guidelines published by Infectious Disease Society of America (IDSA) on prevention of such infections following surgery.
Brachial plexus neuropraxia
Ikeda et al. describes that careful positioning of arm of lesion side prevents injury to brachial plexus during surgery (26). Diagnosis of injury to brachial plexus can be confirmed by EMG. Detection and prevention of positional related neuropathy involves SSEP (11,15). Neuropraxia of brachial plexus presents with diminished reflexes, sensory and motor deficits of the arm. Most of the patients resume function spontaneously, while, others require orthosis/splinting; physical/occupational therapy. Surgery is reserved for refractory cases.
It is important to perform the neck dissection with paying careful attention to the underlying neurovascular structures, while maintaining a complete cancer resection.
Our group has reported its safety and feasibility in a subset of patients with grave’s disease, those having small-sized substernal goiters (27). We have also reported its safety and similar outcomes for postoperative complications when compared to conventional cervical thyroidectomy (9,28). Robotic thyroidectomy has shown same-day discharges in 83.2% of cases while conventional approach shows that in 34.5% (28). However, FDA restrictions have caused a number of North American surgeons to evade the use of the robot-assisted approach.
Conclusions
Robotic surgery has enhanced the surgeon’s capabilities in a very short period of time. We have successfully reported the safety and feasibility of robotic thyroidectomy in management patients with benign and malignant thyroid diseases, as well as in patients with grave’s disease who don’t have large substernal goiters. We have also reported no difference in postoperative complications in patients undergoing robotic thyroidectomy when compared to patients who underwent the conventional cervical approach. Robotic thyroidectomy has been shown to have 83.2% discharge rate on the same day of the procedure, compared to 34.5% in the conventional cervical approach. In spite of studies showing comparable outcomes, the adoption of robot assisted thyroid surgery has been measured in North American. There may be several reasons for this such as the additional training, cost, time associated with achieving proficiency in these techniques. Also, the current FDA restrictions on robot assisted thyroid surgery limit the opportunity for proctored learning and consequently rapid adoption of these techniques.
The future of robotic thyroid surgery and whether it is more widely adopted lies in the ability to standardize care that reliably establishes safe and high quality outcomes that are at least comparable to those achieved with traditional thyroidectomy. Until then, the concept of “do no harm” must be entertained, especially during the learning curve period of these techniques. A balanced investigation and a thorough data analysis are warranted to fully explore the advantages and the disadvantages of this new merging technology by multi-institutional clinical trials and maintaining a data registry.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Miccoli P, Bellantone R, Mourad M, et al. Minimally invasive video-assisted thyroidectomy: multiinstitutional experience. World J Surg 2002;26:972-5. [PubMed]
- Bellantone R, Lombardi CP, Raffaelli M, et al. Central neck lymph node removal during minimally invasive video-assisted thyroidectomy for thyroid carcinoma: a feasible and safe procedure. J Laparoendosc Adv Surg Tech A 2002;12:181-5. [PubMed]
- Abdelgadir Adam M, Speicher P, Pura J, et al. Robotic thyroidectomy for cancer in the US: patterns of use and short-term outcomes. Ann Surg Oncol 2014;21:3859-64. [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach; surgical outcome of 581 patients. Endocr J 2009;56:361-9. [PubMed]
- Ruggieri M, Straniero A, Pacini FM, et al. Video-assisted surgery of the thyroid diseases. Eur Rev Med Pharmacol Sci 2003;7:91-6. [PubMed]
- Lombardi CP, Raffaelli M, Princi P, et al. Video-assisted thyroidectomy: report on the experience of a single center in more than four hundred cases. World J Surg 2006;30:794-800. [PubMed]
- Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64. [PubMed]
- Noureldine SI, Jackson NR, Tufano RP, et al. A comparative North American experience of robotic thyroidectomy in a thyroid cancer population. Langenbecks Arch Surg 2013;398:1069-74. [PubMed]
- Noureldine SI, Lewing N, Tufano RP, et al. The role of the robotic-assisted transaxillary gasless approach for the removal of parathyroid adenomas. ORL J Otorhinolaryngol Relat Spec 2014;76:19-24. [PubMed]
- Park JH, Lee J, Hakim NA, et al. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck 2014. [Epub ahead of print]. [PubMed]
- Landry CS, Grubbs EG, Perrier ND. Bilateral robotic-assisted transaxillary surgery. Arch Surg 2010;145:717-20. [PubMed]
- Katz L, Abdel Khalek M, Crawford B, et al. Robotic-assisted transaxillary parathyroidectomy of an atypical adenoma. Minim Invasive Ther Allied Technol 2012;21:201-5. [PubMed]
- Lee S, Ryu HR, Park JH, et al. Early surgical outcomes comparison between robotic and conventional open thyroid surgery for papillary thyroid microcarcinoma. Surgery 2012;151:724-30. [PubMed]
- Wilson MN. Modification of two-incision trans-axillary robotic thyroidectomy. J Robot Surg 2014;8:325-327.
- Berber E, Siperstein A. Robotic transaxillary total thyroidectomy using a unilateral approach. Surg Laparosc Endosc Percutan Tech 2011;21:207-10. [PubMed]
- Walvekar RR, Wallace E, Bergeron B, et al. Retro-auricular video-assisted "gasless" thyroidectomy: feasibility study in human cadavers. Surg Endosc 2010;24:2895-9. [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636-41. [PubMed]
- Terris DJ, Singer MC. Robotic facelift thyroidectomy: Facilitating remote access surgery. Head Neck 2012;34:746-7. [PubMed]
- Perrier ND, Randolph GW, Inabnet WB, et al. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid 2010;20:1327-32. [PubMed]
- Park JH, Lee CR, Park S, et al. Initial experience with robotic gasless transaxillary thyroidectomy for the management of graves disease: comparison of conventional open versus robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 2013;23:e173-7. [PubMed]
- Lee HY, Richmon JD, Walvekar RR, et al. Robotic transoral periosteal thyroidectomy (TOPOT): experience in two cadavers. J Laparoendosc Adv Surg Tech A 2015;25:139-42. [PubMed]
- Terris DJ, Snyder S, Carneiro-Pla D, et al. American Thyroid Association statement on outpatient thyroidectomy. Thyroid 2013;23:1193-202. [PubMed]
- Jeannon JP, Orabi AA, Bruch GA, et al. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [PubMed]
- Jansson S, Tisell LE, Hagne I, et al. Partial superior laryngeal nerve (SLN) lesions before and after thyroid surgery. World J Surg 1988;12:522-7. [PubMed]
- Watkinson JC. Fifteen years' experience in thyroid surgery. Ann R Coll Surg Engl 2010;92:541-7. [PubMed]
- Ikeda Y, Takami H, Niimi M, et al. Endoscopic thyroidectomy and parathyroidectomy by the axillary approach. A preliminary report. Surg Endosc 2002;16:92-5. [PubMed]
- Jackson NR, Yao L, Tufano RP, et al. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck 2014;36:137-43. [PubMed]
- Noureldine SI, Abdelghani R, Saeed A, et al. Is robotic hemithyroidectomy comparable to its conventional counterpart? Surgery 2013;154:363-8. [PubMed]