Effect of young age, positive margins, and triple negative status on disease recurrence after breast conserving therapy
Introduction
Breast conserving surgery and mastectomy are both widely accepted surgical treatments for breast cancer. Where both apply, survival probabilities after surgery are regarded as equivalent (1-5). However, the risk of local recurrence is currently slightly greater after breast conserving surgery despite the addition of whole breast irradiation, although the difference is becoming smaller and clinically insignificant (6-8). This risk is on average 0.5% to 1% per year (1-11), but the highest hazard occurs during the first 5 years after surgery (12).
Breast cancer recurrence after breast conserving surgery should be preventable to some extent. Effects of certain risk factors which are associated with disease recurrence might be modified to help reduce the risk of recurrence (12-22). We need consistent and valid information especially on those factors whose effects are modifiable.
Several factors have been consistently found to be associated with an increased risk of breast cancer recurrence after breast conserving surgery. Among them must include inadequate surgical margins (12-16), young age (17-20), and certain immunohistochemical markers of breast cancer subtypes (16,21-24). In the present study, we investigate the effects of all these risk factors on breast cancer recurrence after breast conserving surgery in a group of South-East Asian women.
Materials and methods
Medical records of breast cancer patients who underwent breast conserving surgery within the 10-year period from 2001 to 2010 were reviewed. The study was approved by the Hospital’s Research Ethics Committee. We collected information on age; histological type, size, grade, and histochemical analyses of the tumor including hormonal and Human Epidermal Growth Factor Receptor-2 (HER-2) expressions; axillary lymph node metastasis; TNM stage; type and number of operations; surgical margins; and adjuvant treatment including whole breast irradiation, chemotherapy and endocrine treatment. The outcome data collected included cancer recurrence, site of recurrence, and death from any cause, as well as the time interval from surgery to each outcome.
Width of the margins of surgical specimens, measured from the approximate tumor boundary to the resection edge, was determined by pathologists, microscopically if necessary. Six margins were routinely measured. Close surgical margin was defined as at least one free margin width of 1 mm or less. Involved or positive margins were defined as one or more margins at least not microscopically free from cancer. Final margins referred to margin status after reoperation, if done for whatever reason. Thus if the initial margin was involved, but a re-excision or mastectomy was done thereafter and all margins made free, then the final margin status was free. If no reoperations were done, then the initial and final margin status would be the same.
Young age was defined as 35 years or less. Disease recurrence was defined as any breast cancer arising or detected in the body after treatment of the primary disease, with the exception of contralateral breast cancer (25). Locoregional recurrence was defined as a disease recurrence arising in-breast (ipsilateral) or in ipsilateral regional nodes. Distant recurrence was defined as a recurrence outside of the breast and regional nodes. Contralateral breast cancers in the absence of distant metastasis were treated as another primary cancer. All types of recurrences as defined were not exclusive of one another. Thus locoregional recurrence could occur simultaneously with distant recurrence.
Quantitative data were summarized as mean and standard deviation, or median and range as appropriate. Categorical data were summarized as counts and percentage. Survival and recurrence probability were estimated using the Kaplan-Meier or Nelson-Aalen methods. Risk factors for recurrence were identified using Cox proportional hazards models. All statistical analyses were performed using Stata version 12 (Stata Corp, College Station, TX, USA) statistical software. Two-tailed P values ≤0.05 were considered statistically significant.
Results
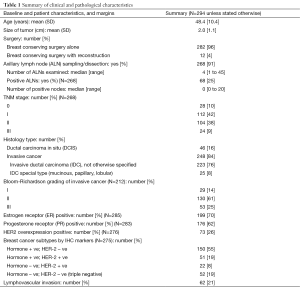
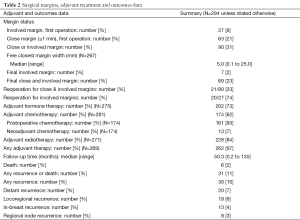
We selected 290 women during the study period who had complete medical records. There were four women who had operations on both breasts. Thus, a total of 294 breasts were included in the present analysis. There were missing values for some of the risk factors considered in the present study; the risk factor used in subsequent analyses with the largest proportions of missing values had 15% of the values missing. These missing values occurred during the period where routine measurements of risk factors such as HER2/neu and Ki67 expressions were not done. A summary of the characteristics of patients and their tumors are presented in Table 1. Outcomes of treatment are presented in Table 2.
Full table
Full table
Patients in the present series were on the average 48 years old, with an SD of 10 years, and 9% (27/294) were aged 35 years or younger—a rather young population. The size of the tumors found at operation was only 2 cm on average, with axillary lymph node involvement in 25% of those evaluable. The overwhelming number of patients had early breast cancer, with TNM stages II or less in 91% of the total. Non-invasive cancers constituted 16% of all cases. Patients with more advanced cancers underwent neoadjuvant chemotherapy prior to breast conserving surgery, specifically for the purpose of breast conservation. These patients constituted only 7% of the total. Tumor grade was missing for a relatively large number of patients; hence this risk factor was not used in subsequent analyses.
Surgical margins as evaluated by pathologists were initially close in 63 (21%) and not free from cancer (involved margins) in 27 (9%) of all initial operations. Reoperations were done in 21 cases, 20 for involved margins, and 1 for close margins. Of the seven patients who had final involved margins, all refused reoperation, and whole breast irradiation was given in five, while one refused radiotherapy, and one was lost to follow-up. Thus, the final margin status was free and not close in 225 (77%), free but close in 62 (21%) and involved in 7 (2%).
Hormone receptor (HR) positive cancers [estrogen receptor (ER) or progesterone receptor (PR) positive, or both] constituted 73% of the total. HER2 positive cancers were seen in 26% of cases, a large proportion but typical of Asians. Triple negative cancers, defined as cancers with both hormonal receptors (HRs) and HER2 negative immunohistochemical stains, were found in 19% of cases. The majority of patients (over 55%) had hormone-positive, HER-2 negative cancers.
Most patients were treated according to accepted guidelines, but some refused to comply with some treatment modalities. Younger patients were usually treated in a similar manner as older patients, given identical conventional prognostic and predictive markers, although there was a tendency towards more aggressive treatment in equivocal cases.
After a median follow-up time of 50 months (4.2 years), with a maximum follow-up time of 135 months (11.3 years), there were 30 recurrences overall (10% of 294), of which 19 (63%) included locoregional recurrence, and 20 (67%) included distant recurrence. There were 13 in-breast (ipsilateral breast) recurrences (4% of 294), constituting 43% of all recurrences. There were six deaths, with five due breast cancer and one due to other causes. Thus, the disease-free survival was 82.5% (95% CI: 74.8% to 88.1%) at 10 years, while the overall survival was 95.5% (95% CI: 89.5% to 98.2%) at 10 years. The cumulative in-breast recurrence rate was 9.3% (95% CI: 4.9% to 17.2%) at 10 years, averaging 0.93% per year.
Univariable Cox proportional hazards regression modeling focusing on all disease recurrences as the outcome revealed several statistically significant risk factors. But on multivariable analysis, only young age, tumor size, number of positive axillary nodes, other than hormone positive, HER-2 negative status, (“non-Luminal A”) and involved surgical margins were independently and significantly related to all recurrences (Table 3). Identical set of factors was also found in the subset of patients who did not undergo reoperation.

Full table
If only locoregional recurrence was considered, on multivariable Cox regression analysis, young age, tumor size, involved surgical margin, and whole breast irradiation were independently and significantly related to the outcome (Table 4). The latter factor reduced the recurrence risk. We also looked at the influence of margin width on locoregional recurrence, but could not find a significant association, even on univariable analysis. Subtypes of cancer had no evident effect on locoregional recurrence.

Full table
If the focus was on distant recurrence as the outcome, young age was no longer a statistically significant risk factor in a multivariable Cox regression analysis. Thus, the effect of tumor size, number of positive axillary nodes, involved surgical margin, and triple negative receptor status could explain away the effect of young age on distant recurrence (Table 5).

Full table
An analysis using in-breast recurrence as the outcome was similar to that using locoregional recurrence as the outcome, and young age was an important risk factor. Similarly, an analysis considering only invasive cancers (excluding purely non-invasive cancers) yielded identical results as above. An analysis using 40 years as the cutoff for young age showed identical results to that using 35 years as the cutoff. Finally, an analysis including year of diagnosis categorized as before 2008 (170 observations, 58%) and 2008 and after (124 observations, 42%) as an additional risk factor, a cutoff chosen because we started doing more oncoplastic surgery at this time, as well as using newer systemic adjuvants such as trastuzumab, there was no evident effect of year of diagnosis on disease recurrence. Hence all these analyses were not described in detail in the present report.
When we excluded patients with stage III disease (24 observations, 9%) from the analysis, to see whether the results of the analysis would apply to early breast cancer patients, there were some interesting observations. First note than stage III disease in the present study, as may be expected, was significantly related to axillary lymph node metastasis, to triple negative disease, and disease recurrence, though not related to age. Thus, in this subgroup analysis, young age and involved margins were still significantly related to locoregional and overall recurrence, but the number of positive axillary nodes was no longer significant, because patients had fewer positive nodes. Similarly, triple negative disease, as well as positive axillary nodes, was no longer significantly related to distant metastasis in this early stage breast cancer subgroup. All estimates were also much less precise because of the reduced number of outcomes.
Discussion
The present study focused on all breast cancer recurrence because there were very few deaths in the series, and almost all deaths were preceded by distant recurrence. Since most recurrences involved the ipsilateral breast and regional nodes as well, we also examined locoregional recurrence and related risk factors. Separate analyses for distant recurrence were also done, as risk factors may differ for different types of recurrence.
Ipsilateral breast cancer and regional node recurrence following breast conserving surgery is a distressing event and has an impact on subsequent survival (4,5,12,26). After locoregional recurrence, the conserved breast is often completely removed (5), and further adjuvant or a change in adjuvant therapy might be needed (27), adding significantly to the adverse consequences if and when the disease recurs. Hence prevention of locoregional disease recurrence is an important goal of breast cancer treatment.
All recurrence, as well as survival, probabilities found in the present study compared favorably to those of other studies. Randomized controlled trials, mostly conducted a few decades ago, showed a locoregional or in-breast recurrence rates of less than 1% per year (1-3). More recent studies, both randomized trials and observational studies, showed recurrence rates less than 0.5% per year (6-9), due to improved locoregional and systemic therapy, but mainly the latter (6,28).
Young age is a known and important risk for breast cancer recurrence, as was found in the present study. This is believed to be partly due to a biologically distinct behavior of breast cancers occurring in this age group (18,29,30). These cancers are often aggressive and tend to recur locally as well as distantly, despite polychemotherapy, endocrine therapy and whole breast irradiation (17,18,20,30,31). Moreover, young breast cancer patients tend to harbor more triple-negative disease (18,31). In the present study, young age was mainly a risk factor for locoregional recurrence. Several studies seemed to support this result as well (3,30,32,33). Newer targeted therapy in combination with conventional treatment might help control these cancers more successfully.
Surgical margin status after breast conserving surgery is also an established risk factor for local recurrence, specifically in-breast recurrence (14-16). The most recent consensus guidelines, which included a review of all available and relevant evidence, concluded that free margins of any width are adequate for most patients, but a positive margin requires reoperation to achieve negativity (13,14). In the present study, we could not identify a significant association between margin width and recurrence of any type if the margins were free. However, a positive margin which was not treated with further surgery was strongly associated with locoregional as well as distant recurrence (26), suggesting a need for reoperation in these cases, consistent with recent guidelines.
Breast cancer subtypes based on HR and HER-2 expression phenotypes have been shown to correlate with disease recurrence (21,22). One particular subtype, the triple negative breast cancer, is a well-documented risk for locoregional and distant recurrence (8,22,23,34). It is also an important risk factor in the present study, but only for distant recurrence. This result seems to suggest that triple negative status could impact distant recurrence more than locoregional recurrence, at least for patients with more advanced disease, as was similarly found by other studies (22,23,34).
The size of the tumor is also a well-known risk factor for recurrence (7,12). The larger the tumor, the more likely that undetected residual microscopic, or multifocal, or multicentric, tumors are left in the conserved breast (12,35). Even breast irradiation cannot eliminate all risks, especially if radioresistant cells are present (36,37). However, systemic treatment can affect locoregional control as well (35). Because the size of the tumor and number of positive lymph nodes were already more strongly associated with recurrence, TNM staging as a risk factor, which is essentially a categorization of tumor size and number of lymph nodes, failed to reach statistical significance in the present study.
Axillary lymph node status is the single most important and a classic prognostic marker of breast cancer recurrence and survival (2,3,12). The present study found no evidence to contradict this fact. The number of positive axillary nodes was associated with all recurrence (which included distant recurrence as well) and distant recurrence, rather than just locoregional recurrence (32), emphasizing the role of regional nodes as a marker of distant spread.
So what is novel about these findings? We believe the results of the present study add to an enlarging pool of consistent knowledge of risk factors concerning recurrence after breast conserving surgery—albeit one from a South-East Asian country. The strength and consistency, or robustness, of the associations between risk factors and disease recurrence—despite the small number of recurrences—cannot be explained by bias, or pure chance. Our evidence points towards young age as a risk for locoregional recurrence, while triple negative status was related to distant recurrence. Surgical margin status, however, was associated with all recurrences, regardless of whether locoregional or distant.
In an analysis not shown in the present report, we found that, after adjusting for tumor size, margin status, and tumor subtype, the combination of young age and post-operative whole-breast irradiation was associated with similar locoregional disease-free probability to that of the combination of older age and no radiation.
Some of the clinical implications of the present study are straightforward. To reduce the risk of breast cancer recurrence, all surgical margins must be free after breast conserving surgery (13,14), and, especially for younger women, widely free (31,37,38). Young breast cancer patients also require whole breast irradiation (12,15,17,37). More aggressive or more use of additional adjuvant treatment, such as newer targeted therapy, longer duration of endocrine treatment (39), and ovarian suppression (40), should be considered in the young in view of the increased risk in locoregional recurrence, which would impact the long-term overall survival of these patients. It might be suggested that the use of neoadjuvant chemotherapy in patients with larger tumors could be of some value even if the tumor is operable, to reduce tumor size and number of positive axillary lymph nodes prior to breast conserving surgery (41). This way, the risk of disease recurrence might be reduced as well. Finally, triple negative tumors are a major subject of therapeutic research, and more targeted therapies are on the horizon to address the problem.
Conclusions
We present further data supporting young age, triple negative status, and surgical margin status as important risk factors for disease recurrence after breast conserving surgery for breast cancer. Although the sample size and number of outcomes in our study were small, the associations were strong enough to be detectable. Other important risk factors detected included size of the tumor, number positive nodes, and whole breast irradiation. Modifying the effects of these important risk factors could result in a lower probability of disease recurrence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [PubMed]
- Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 2006;24:2028-37. [PubMed]
- Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 2009;27:2466-73. [PubMed]
- Cabioglu N, Hunt KK, Buchholz TA, et al. Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer 2005;104:20-9. [PubMed]
- Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol 2013;20:3469-76. [PubMed]
- Wang J, Xie X, Wang X, et al. Locoregional and distant recurrences after breast conserving therapy in patients with triple-negative breast cancer: a meta-analysis. Surg Oncol 2013;22:247-55. [PubMed]
- Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-32;discussion 432-3. [PubMed]
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011;103:478-88. [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507-15. [PubMed]
- Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010;46:3219-32. [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [PubMed]
- Pilewskie M, Ho A, Orell E, et al. Effect of margin width on local recurrence in triple-negative breast cancer patients treated with breast-conserving therapy. Ann Surg Oncol 2014;21:1209-14. [PubMed]
- Antonini N, Jones H, Horiot JC, et al. Effect of age and radiation dose on local control after breast conserving treatment: EORTC trial 22881-10882. Radiother Oncol 2007;82:265-71. [PubMed]
- Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer 2012;48:3355-77. [PubMed]
- Gentilini O, Botteri E, Rotmensz N, et al. Breast-conserving surgery in 201 very young patients (<35 years). Breast 2010;19:55-8. [PubMed]
- Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: A systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 2015;24:175-81. [PubMed]
- Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008;26:2373-8. [PubMed]
- Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831-41. [PubMed]
- Gangi A, Chung A, Mirocha J, et al. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg 2014;149:252-8. [PubMed]
- Keating NL, Landrum MB, Brooks JM, et al. Outcomes following local therapy for early-stage breast cancer in non-trial populations. Breast Cancer Res Treat 2011;125:803-13. [PubMed]
- Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007;25:2127-32. [PubMed]
- Meric F, Mirza NQ, Vlastos G, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer 2003;97:926-33. [PubMed]
- Shen J, Hunt KK, Mirza NQ, et al. Predictors of systemic recurrence and disease-specific survival after ipsilateral breast tumor recurrence. Cancer 2005;104:479-90. [PubMed]
- Livi L, Paiar F, Saieva C, et al. Survival and breast relapse in 3834 patients with T1-T2 breast cancer after conserving surgery and adjuvant treatment. Radiother Oncol 2007;82:287-93. [PubMed]
- Colleoni M, Rotmensz N, Peruzzotti G, et al. Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol 2006;17:1497-503. [PubMed]
- Bollet MA, Sigal-Zafrani B, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol 2007;82:272-80. [PubMed]
- Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer 2014;17:301-7. [PubMed]
- Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer 2006;106:35-41. [PubMed]
- de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer 2006;42:351-6. [PubMed]
- Braunstein LZ, Niemierko A, Shenouda MN, et al. Outcome following local-regional recurrence in women with early-stage breast cancer: impact of biologic subtype. Breast J 2015;21:161-7. [PubMed]
- Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer--bigger is not better. N Engl J Med 2012;367:79-82. [PubMed]
- Poortmans PM, Collette L, Horiot JC, et al. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol 2009;90:80-5. [PubMed]
- Chen W, Sonke JJ, Stroom J, et al. The effect of age in breast conserving therapy: A retrospective analysis on pathology and clinical outcome data. Radiother Oncol 2015;114:314-21. [PubMed]
- Mazeh H, Sagiv I, Katz D, et al. Association between patient age, volume of breast tissue excised, and local recurrence. J Surg Res 2013;181:187-92. [PubMed]
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. [PubMed]
- Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436-46. [PubMed]
- Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res 2012;14:R83. [PubMed]


