Extended thoracodorsal artery perforator flap for breast reconstruction
Introduction
Conservative mastectomies lead tovarying amounts of volume deficit depending on the dimensions of the resected tissue. De-epithelialized flaps from the lateral thoracic wall and the back can be transposed to the anterior thorax for breast mound reconstruction using the thoracodorsal artery perforator (TDAP) flap or the lateral intercostal artery perforator (LICAP) flap.
The TDAP flap was originally described in 1992 (1) as a method of harvesting the skin and subcutaneous island of the traditional latissimus dorsi musculocutaneous (LD-MC) flap without the muscle. It was reported as a possible breast reconstruction method in 1996 (2), and Hamdi published its first clinical use for breast reconstruction in 2004 (3). Several studies have demonstrated that the TDAP flap is a reliable and safe technique (4-6).
The TDAP flap is irrigated by the proximal perforator of the descending branch of the thoracodorsal artery (Figure 1). This branch is consistently present, according to several anatomical studies (7-13). The superior (scapular) and inferior (lumbar) fat compartments can be partially captured and irrigated by this proximal muscle perforator in the same manner as in the extended LD-MC flap (14) when additional volume is needed without the muscle (Angrigiani 2010 “TDAP Flap” presented at 13th International Perforator Flap Course Mexico 2010). We have named this variation “the extended TDAP flap” (Figure 2).
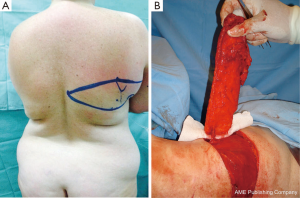
With this method, sufficient volume to reconstruct a B-cup sized breast can be obtained using a totally or partially de-epithelialized flap.
Additional fat grafting may be performed to increase volume in the same procedure with the use of the TDAP flap as a scaffold by lipofilling (Figure 3).
Surgical technique
Evaluation of volume deficit and location
The best way to evaluate needed volume need is to weight or measure the resected tissue. In secondary or delayed reconstruction, however, the volume is frequently underestimated as the retracted tissue may mislead the calculation. Intraoperative evaluation after releasing the retractive scar exposes the true volume deficit. Contralateral comparison, if possible, may provide a good approximation.
Flap indication
The indications for the TDAP flap, which are similar to those of the LD-MC flap, are as follows: primary or additional volume for breast reconstruction; salvage procedure for exposed implants; primary or additional surface (envelop) reconstruction; and combined implant autologous tissue reconstruction. Selection of a regular LD-MC flap or a TDAP flap must be done prior to designing the flap. There are some differences in the designs of these two flaps; these are described in the next paragraph. The LD flap involves muscle harvesting, is easier to perform, and requires shorter operating time. The TDAP flap involves a more complex technique but preserves the muscle and is associated with less seroma in the donor area.
Once the volume deficit is known, possible donor areas must be evaluated. A pinch test gives an approximate idea of the amount of subcutaneous tissue available for transfer as an island flap. It is important to measure the middle back; the lumbar and subscapular fat pads can be transferred partially. Of the available fat flap volume, 50% may be safely added by lipotransfer, using the flap as a scaffold for flap survival.
The volume of the back should be compared with that of the lateral thoracic wall. In cases with available volume in the lateral thoracic area, an intercostal perforator flap should be considered, although the same area can be safely harvested with the TDAP flap.
Flap design
The flap is designed with the patient in the standing position, with the arms at the sides and the hands on the waist. The patient is asked to actively contract her back muscles, at which time the anterior lateral border of the LD muscle appears clearly under the skin and is marked with a line. The absence of this obvious contraction in mastectomy sequelae cases is highly suspicious of a neurovascular muscle pedicle lesion. Although representative of only a nerve lesion, it is frequently associated with a vascular lesion. The possibility of raising a LD-MC flap with “compensatory” irrigation has been reported, but we prefer to utilize other options to avoid the possible risk of flap loss.
A point “A” is marked on the anterolateral muscle line, 8 cm below the axillary fold. The descending branch of the proximal perforator artery runs parallel and approximately 2 cm lateral to that line. The proximal perforator branch of the descending thoracodorsal artery branch pierces the muscle in the line of the descending branch, at 8 cm or more from the axillary fold. However, in 20% of the cases, a direct cutaneous branch from the descending branch of the thoracodorsal artery is the most important cutaneous branch (based on diameter). This direct cutaneous branch does not pierce the muscle; instead, it passes immediately anterior to the lateral border of the muscle. Thus, the design of the flap must exceed the edge of the muscle to assure the presence of this branch in the raised flap. This is the main difference with the LD-MC flap; the skin island of the LD may be designed more posterior or inferior without including point “A” within the flap design. It can be safely nourished by other muscle perforators of the thoracodorsal artery, resulting in a more posteriorly placed final scar.
The piercing point of the perforator (or cutaneous branch) must be included in the flap design as its irrigation is necessary. The flap length reaches the union of the lateral 3/4 with the most medial quarter of the back. Achieving the maximum possible length can improve insetting in the breast mound. Clinical criterium is important for resection of the distal, under irrigated part of the flap, when it is fully elevated. No method has been reported previously for assessing this distal area. In addition, in our experience, it varies greatly across patients. The dimension of this distal under-perfused area is not related to only the perforator diameter; the subcutaneous vascular network status might also play an important role in the functional and physiological irrigation of the flap.
The flap width is designed according to the possibility of direct closing of the donor site. The skin and the associated subcutaneous tissue are pinched with the thumb and index finger to mark the desired width. It is preferable to achieve a fine aesthetically acceptable scar than a skin graft in the donor area. The flap length extends across the width of the back when the design is horizontal or across the supero-inferior angle of the scapula when the design is made obliquely upward. We prefer the oblique design because the thickness of the adipose tissue in the parascapular area provides more volume. However, many patients prefer the horizontal design. The final choice depends on the patient’s decision.
The location of the perforators is ideally determined using preoperative angiography and color Doppler ultrasonography. When these techniques are not available, the surgeon must rely on anatomical knowledge and clinical experience in using the flap to locate these vessels, which in most cases, are in an area 8-cm below the axillary fold.
Single and double flap harvesting
De-epithelialized TDAP may be applied in unilateral or bilateral cases, with variations in surgical technique depending on the case. In unilateral cases, the patient is placed in contralateral decubitus, with the arm prepared free hold by an assistant. This position allows easy access to the pedicle origin and direct transfer of the flap to the anterior thorax. In bilateral cases, the patient is placed in ventral decubitus, and the procedure is performed by two teams simultaneously.
The flap is raised in the distal to proximal direction, superficial to the deep fascia, while observing the fascia of the LD muscle. The perforator arteries are carefully observed, under 4× magnifications. Continuous and progressive control of the bleeding quality from the end of the flap is an excellent way to monitor the presence of a good perforator. If the flap has excellent perfusion by the time it is half separated from the LD muscle, the perforator is likely to be adequate (diameter >0.5 mm). By contrast, if the perfusion markedly decreases when the flap is half-raised and the medial intercostal perforators are sectioned, we would prefer to postpone the procedure. Such a situation was not observed in this series.
When an “extended TDAP” is planned, the subcutaneous tissues superior and inferior to the skin incision are harvested. Lipofilling of this flap may supplement the final volume. We usually add 150 cc.
Dissection continues along the suprafascial plane to the anterior border of the muscle and proceeds superiorly up to the perforator entrance point. Locating the lateral edge of the muscle is important because the descending thoracodorsal artery branch runs parallel to that edge, at a distance of ≤2-4 cm. Therefore, the proximal perforator is found at approximately the same distance from the edge. In cases involving a direct cutaneous branch, this level is at the edge surrounding the muscle.
The proximal perforator artery also has an accompanying vein. Once this artery has been located, we perform complete dissection of the skin around the island itself. If the flap has good vascularization (bleeding from the skin edges and skin refilling), and no perforator is apparent when the lateral anterior border of the muscle is completely exposed, the direct cutaneous branch of the thoracodorsal artery should be carefully looked for. If it is not present, or is of a small diameter, then the lateral intercostal perforator must be present and is the main irrigating source of this flap. This rationale should be applied if the flap is well vascularized after passing the anterolateral border of the LD muscle. Neighboring cutaneous arteries may be of different calibers: if one has a large diameter, the other one is smaller, or vice versa, to compensate for the necessary blood flow of the skin. This was originally described by the French anatomist Dubreuill Chambardell, reported by Salmon (15). If the flap turns white or bluish, suggesting sluggish circulation, presence of a lesion of the muscle perforators must be assumed, and the flap should be discarded.
Once the perforator is completely exposed, there are several possibilities for continuing the surgical procedure, as described below:
- Propeller flap. The dissection around the perforating artery is minimal and serves to release the muscle and allow flap rotation along this axis, creating the “flap helix” (propeller) (16,17). The procedure is simple and quick. A special dissection technique is not required. The main disadvantage is its shorter length. The flap does not reach the midline of the anterior chest wall. A substantial portion of the flap remains in the subaxillary area, where it is not necessary, while the medial portion of the breast does not receive adequate volume. If a longer flap is harvested to reach the medial part of the breast, tissue suffering as well as steatonecrosis might be observed.
In cases of mastectomy sequelae, we release the scar and leave a gap to place the flap. The previous scar incision is made continuous with the flap incision. In immediate reconstructions, when performing skin-sparing mastectomy or when no scar at the breast side is present, the flap is de-epithelialized and tunneled, remaining under the skin below the tunnel. Donor site closure is performed in two planes. A suction drain is placed and removed 48-72 hours after surgery. - Flip-over flap. The flap is raised in the same conventional manner, from distal to proximal. Once the muscle perforators corresponding to the descending branch of the thoracodorsal artery are visualized, the dissection is discontinued. The flap is de-epithelialized and turned over the anterior part of the thorax. This “turn over” or “flip-over” flap is very simple to harvest. There is an important portion of the flap volume that remains under the axillary area and lateral to the breast. It provides a good volume for reconstruction of a medium-sized breast or complements a partial mastectomy repair.
- Muscle-sparing flap. The flap is raised in the distal to proximal direction; once the lateral border of the LD is approached and the perforators of the descending branch are visualized, the muscle containing the perforators is sectioned-muscle sparing technique (18); the flap can be turned over or rotated to the breast area. This technique is a variation of the propeller but it partially damages the muscle innervation and has a reduced reaching point. It is used for partial volume deficit reconstruction on the lateral aspect of the breast.
- Conventional TDAP flap. In this procedure, the perforator is dissected free from the muscle, and the flap is tunneled under the most lateral muscle fibers to completely preserve the muscle innervation. Although this is a somewhat more cumbersome and difficult procedure, it affords the greatest pedicle length. The flap reaches the thoracic anterior midline, allowing better flap insetting and positioning of the fat volume. The main disadvantage of this method is the necessity for magnification and specialized instruments. The flap is then deepithelialized. We frequently leave a small cutaneous island to monitor flap viability.
Treatment of donor area
The donor area is closed directly in two layers. Vicryl and monocryl internal sutures are utilized for approximation of wound edges, and interrupted 4-0 nylon is used for the skin. Suction drainage is usually applied for 24-48 hours, but we leave them in place as long as necessary. They are removed when there is no more drainage. As the LD is not mobilized in this technique, wound drainage is generally moderate. It is greater in the cases of “extended TDAP” than in regular TDAP, as there is less undermining of the wound flaps.
Flap transference
In unilateral cases, the flap is transferred directly. When the flap incision is not in continuity with the breast wound, a tunnel is performed under the lateral breast mound and lateral thoracic wall for passage. The flap is left without final insetting; the back wound is covered, and the patient is turned to dorsal decubitus position, at which time the anterior area is prepared again.
Insetting
The flap is distributed under the breast, enveloped and fixed at the borders with interrupted absorbable sutures. If the nipple-areolar complex must be reconstructed, a round skin island, 6 cm in diameter, is left in the flap. In cases of adenomastectomy sequelae, when the nipple-areolar complex has been preserved, a small skin island is preserved to monitor the flap viability. It is usually placed in the breast submammary sulcus and is eventually removed during a complementary procedure.
Clinical experience
A total of 45 patients underwent partial or complete autologous tissue breast reconstruction from 1996 to 2014 with a TDAP. There were two cases of complete failure due to technical errors and four cases of partial distal tissue suffering or necrosis due to exaggerated flap length. These four cases required partial flap resection, or eventually, complete resection and reconstruction with a new flap. Of the total flaps, 39 survived completely. A simple satisfaction level survey of these patients indicated that 32 of these patients were satisfied with the procedure (82%).
Clinical examples
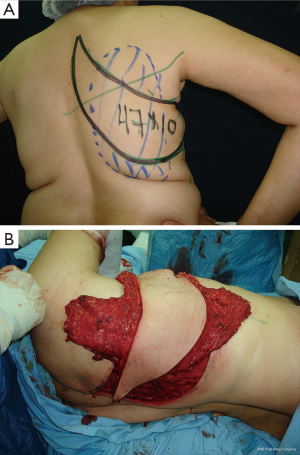
Case 1 (Figure 4): a 43-year-old female patient was scheduled for skin-sparing mastectomy of the left breast. She had previously undergone a lumpectomy on the superolateral quadrant and lymph node biopsy. Autologous tissue reconstruction was performed with a partially de-epithelialized TDAP flap, harvested with an ascending oblique design. This design is similar to the Hammond-type design used for conventional LD-MC flaps. The patient was dissatisfied with the final scar in the donor area but considered the result of the breast shape and volume to be very good. Patient did not continue treatment for nipple-areolar reconstruction or possible complementary aesthetic procedures.
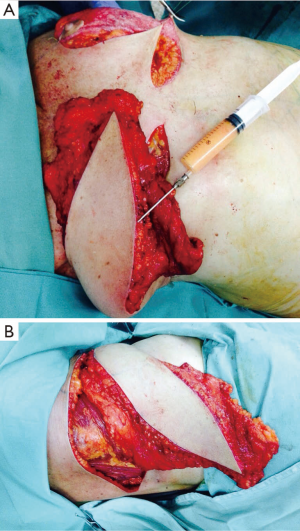
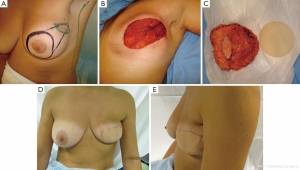
Case 2 (Figure 5): a 42-year-old female patient was scheduled for skin sparing mastectomy (SSM) with extension to the superolateral pole to involve the biopsy area within the resection. The patient had 275 cc silicone implants placed several years prior, which were explanted at the same procedure. The contralateral right implant was left in place. An immediate reconstruction was performed with an extended TDAP, partially de-epithelialized, with no implant. It is possible to compare the final immediate volume obtained with the contralateral side that still has a 275 cc implant. The patient was satisfied with the result. She eventually underwent explantation of the contralateral implant, as advised by the oncologist.
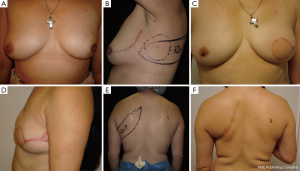
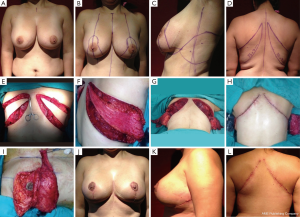
Case 4 (Figure 6): a 41-year-old female patient was scheduled for a bilateral siliconoma resection. A skin-reducing mastectomy was planned with an inverted T pattern and an inferior pedicle. A bilateral de-epithelialized TDAP flap with an ascending oblique design was performed for volume replacement. The flaps were elevated simultaneously by two operating teams that reduced the operating time significantly. The flap was transferred with a flip-over technique (19). The patient was completely satisfied with the final result and the donor area.
Discussion
Autologous tissue breast reconstruction is considered a reliable surgical technique. The LD-MC flap has been the “workhorse” for treating difficult or complicated cases as well as for primary reconstruction. Lipotransference to the conventional LD-MC flap has been reported to increase its initial volume and improve autologous breast reconstruction (20,21). Morbidity of the donor area might be considered a disadvantage, albeit to a minimal extent, for this procedure. Muscle harvesting remains controversial, with conflicting favorable and negative reports on the technique. As mentioned above, the final scar of the LD flap may be placed more posteriorly with an adequate horizontal design. The TDAP design must incorporate the first perforator resulting in a more anteriorly placed final scar.
The incorporation of the TDAP flap, a derivation of the perforator flap era and which was initially described as “the LD-MC flap without muscle”, permits harvesting of the same skin and subcutaneous tissue area normally obtained with the conventional LD-MC flap without the muscle, thereby avoiding the possible morbidities of this procedure. The presence of the muscle might be considered important considering the necessity of volume for the reconstruction. However, the most voluminous part of the muscle remains under the axilla after transferring the flap to the anterior area. The muscle transferred to the breast mound is quite thin, with minimal volume contribution.
The incidence of seroma is almost none in regular TDAP; it is slightly higher in extended TDAP due to the necessary undermining of the donor area but lower compared to the LD flap. In addition, it is not associated with any impairment of shoulder motion. No aesthetic sequelae at the anterolateral border of the muscle on the lateral side are evident in normal-weight women. Steatonecrosis, distal tissue necrosis, and distal tissue suffering are the most common complications. They can be avoided by adequate resection of the distal part of the flap until healthy red bleeding is observed from the dermis. Medial breast volume reconstruction requires a conventional thoracodorsal perforator flap with full dissection of the pedicle in order to reach the midline with well-irrigated tissue.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [PubMed]
- Khoobehi K, Allen RJ, Montegut WJ. Thoracodorsal artery perforator flap for reconstruction. 90th Annual Scientific Assembly of the Southern Medical Association, Baltimore, Maryland, November 20-24. South Med J 1996.89.
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [PubMed]
- Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005;116:762-7. [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [PubMed]
- Schwabegger AH, Bodner G, Ninković M, et al. Thoracodorsal artery perforator (TAP) flap: report of our experience and review of the literature. Br J Plast Surg 2002;55:390-5. [PubMed]
- Adler N, Seitz IA, Song DH. Pedicled thoracodorsal artery perforator flap in breast reconstruction: clinical experience. Eplasty 2009;9:e24. [PubMed]
- Thomas BP, Geddes CR, Tang M, et al. The vascular basis of the thoracodorsal artery perforator flap. Plast Reconstr Surg 2005;116:818-22. [PubMed]
- Lin CT, Huang JS, Yang KC, et al. Reliability of anatomical landmarks for skin perforators of the thoracodorsal artery perforator flap. Plast Reconstr Surg 2006;118:1376-86; discussion 1387. [PubMed]
- Heitmann C, Guerra A, Metzinger SW, et al. The thoracodorsal artery perforator flap: anatomic basis and clinical application. Ann Plast Surg 2003;51:23-9. [PubMed]
- Guerra AB, Metzinger SE, Lund KM, et al. The thoracodorsal artery perforator flap: clinical experience and anatomic study with emphasis on harvest techniques. Plast Reconstr Surg 2004;114:32-41; discussion 42-3. [PubMed]
- Schaverien M, Wong C, Bailey S, et al. Thoracodorsal artery perforator flap and Latissimus dorsi myocutaneous flap--anatomical study of the constant skin paddle perforator locations. J Plast Reconstr Aesthet Surg 2010;63:2123-7. [PubMed]
- Schaverien M, Saint-Cyr M, Arbique G, et al. Three- and four-dimensional arterial and venous anatomies of the thoracodorsal artery perforator flap. Plast Reconstr Surg 2008;121:1578-87. [PubMed]
- Germann G, Steinau HU. Breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg 1996;97:519-26. [PubMed]
- Salmon M. Les artères du tronc et du cou. Paris Masson 1933.
- Thomsen JB, Bille C, Wamberg P, et al. Propeller TAP flap: is it usable for breast reconstruction? J Plast Surg Hand Surg 2013;47:379-82. [PubMed]
- Angrigiani C, Rancati A, Escudero E, et al. Propeller thoracodorsal artery perforator flap for breast reconstruction. Gland Surg 2014;3:174-80. [PubMed]
- Saint-Cyr M, Nagarkar P, Schaverien M, et al. The pedicled descending branch muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg 2009;123:13-24. [PubMed]
- Berry MG, Curnier A, Fitoussi AD, et al. The muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg 2009;124:1000-1. [PubMed]
- Delay E, Gounot N, Bouillot A, et al. Autologous latissimus breast reconstruction: a 3-year clinical experience with 100 patients. Plast Reconstr Surg 1998;102:1461-78. [PubMed]
- Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 2007;119:775-85; discussion 786-7. [PubMed]


