What is the evidence behind conservative mastectomies?
Introduction
The Copernican revolution has been validated by the historical randomized controlled trials (RCTs) by Veronesi et al. and Fisher et al. (1,2) leading to breast conservative surgery definition as standard treatment for early breast cancer.
Thanks to breast cancer screening programs and higher levels of breast cancer awareness, breast conservation rates have increased up to 75% (3).
Today mastectomy cannot be avoided for multicentric disease or after local recurrence (LR) following breast conservative treatments. Moreover the wider diffusion of risk-reducing procedures for women identified to be at higher breast cancer risk who have predisposing gene mutations find in mastectomy the best treatment.
All women undergoing mastectomy can take advantage of the many options available for breast reconstruction.
Together with the diffusion of breast reconstructive techniques, several “conservative” approaches in mastectomy have been developed, in order to allow an immediate reconstruction with better aesthetic results.
The modified radical mastectomy (MRM) or non-skin-sparing mastectomy (NSSM) was described by Madden in 1965 (4) and consists in the removal of all breast tissue, preserving both pectoralis muscles, together with the dissection of level I and II axillary lymph nodes.
The SSM was first described by Toth and Lappert in 1991 (5) with the aim of removing the entire parenchymal breast tissue while preserving the overlying skin of the breast envelope and the natural inframammary fold (6).
The traditional SSM also takes into account the excision of the skin overlying superficial tumors as well as previous biopsy entry sites. However, this is not routinely performed by all surgeons (7).
From the concept of SSM the natural evolution was the nipple-areola complex-sparing (NAC-sparing) mastectomy (NSM), requiring removal of nipple-areolar ducts (8,9). Skin flaps should only be 2-3 mm in thickness at the NAC. The technique could be facilitated by hydro dissection (10) and sharp dissection instead of electrocauterization to limit thermal injury and increase NAC preservation rates (9).
The nipple-areolar ducts are commonly sent for frozen section examination of the NAC for residual cancer suggesting removal of the entire NAC (conversion to SSM) if the frozen section is positive to the disease (11,12). Other authors wait for permanent sections and return to the operating room for the removal of the NAC if final pathology results positive (13). Some other groups recommend the use of intraoperative radiotherapy in association with the NSM (14).
Multiple techniques and skin incisions have been described for NSM in order to prevent NAC necrosis that can be a complication of NSM due to the close dissection under the NAC.
During the last decade, SSMs and NSMs have gained widespread acceptance and are currently considered standard treatment for early breast cancer.
We would like to investigate the evidence behind this radical shift towards conservative mastectomies, where there has been a renewed interest worldwide (15).
NAC-sparing mastectomy would appear to be the most ideal mastectomy alternative, but are we sure it achieves oncological equivalent outcomes when compared to traditional (modified radical) mastectomy and breast conserving approaches? Are women asking for a conservative mastectomy well-informed about the risks and potential adverse outcomes?
Methods
Any RCT comparing a “conservative mastectomy” technique to breast conservative surgery or MRM for the treatment of early-stage breast cancer was considered for inclusion.
In the absence of randomized trials, we considered cohort or case control studies for a narrative description of available evidence.
Our primary outcomes were oncological ones LR and patient-reported outcomes (post-operative quality of life or satisfaction level) as measured by BREAST-Q, EORTC QLQ-BR23 and SF-36. We also considered as secondary outcomes, post-operative short-term complications (infection, hematoma, seroma, skin flaps or NAC necrosis), re-intervention and long-term complication rates and cosmetic outcomes not reported by participants (i.e., evaluation of reconstructive outcomes by the operating surgeon or other uninvolved clinicians).
We performed a review of the English literature by consulting the following databases: Medline, Embase, Cochrane Register of Controlled Trials, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal and Clinicaltrials.gov.
We tried to identify further studies by reviewing reference lists of relevant trials or reviews. A copy of the full article for each reference reporting a potentially eligible study was obtained. When this was not possible, attempts were made to contact study authors to request additional information.
All abstracts identified by the search strategies were screened for duplicates and assessed by two independent review authors to exclude studies that did not meet the inclusion criteria. Disagreements were solved through discussion between two review authors; in cases of persistent disagreement, a third review author was consulted. The full publications of all potentially relevant abstracts were obtained and formally assessed for inclusion. Review authors were not blinded to the names of the study authors, their corresponding institutions and the journal of publication.
A tailored data extraction form was developed to record the details of the studies.
Data was extracted independently by two review authors; differences of opinion between review authors were solved through discussion with a third author. Missing or updated information was obtained by contacting the study authors.
Quantitative data from studies with more than one publication was extracted from the latest source; this was considered as the primary reference.
Results
The search was launched in November 2014. No RCTs comparing NSSM or breast conserving surgery (BCS) versus skin- and NAC-sparing mastectomy (SSM-NSM) were found in literature.
Therefore we only analyzed retrospective series and prospective cohorts (that is level of evidence III and IV) presenting data on LR, post-operative complications and patient satisfaction level.
The high level of heterogeneity between the studies design, stage of disease, tumor characteristics, additional therapies (chemotherapy or radiation therapy), surgical technique, type of reconstruction and follow-up time made it impossible to perform a meta-analysis of the included studies according to LRs, post-operative complications or aesthetic outcomes.
We could only carry out a narrative review of the existing literature, achieving a level III of evidence according to Oxford Classification.
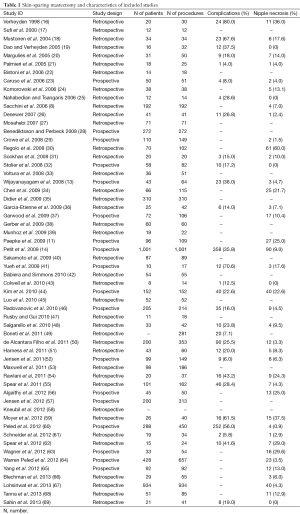
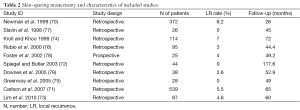
Our review included 58 studies [19 prospective cohorts (34%) and 39 retrospective series (66%)] considering NSM and immediate reconstruction (Figure 1, Table 1) and ten studies [1 prospective cohort (10%) and 9 (90%) retrospective series] considering SSM and immediate reconstruction (70-79) (Table 2).
Full table
Full table
The indications for NSM included invasive cancer, carcinoma in situ and risk-reduction. SSM was performed for carcinoma in situ and invasive breast cancer.
There was high heterogeneity in the inclusion criteria between NSM studies (risk-reducing mastectomy, no NAC involvement confirmed with MRI, no NAC involvement confirmed with intraoperative frozen section, no nipple retraction, bloody discharge or retro areolar microcalcifications, tumor size <3-5 cm, tumor located >1-2 cm from nipple, no skin involvement, no Paget disease, no axillary involvement, BMI <40, no history of collagen vascular disease, small or medium breast size, minimal ptosis, no preoperative irradiation or chemotherapy, no smoking).
Most studies (78%) on NSM were conducted after 2008, confirming that this type of procedure became more popular in the last decade.
Twenty-nine studies in the NSM group reported data on complication rates and 42 studies presented data on NAC partial or complete necrosis (Table 1).
In the NSM group 45 studies and all the studies in the SSM group presented data on LR (Table 2).
Fifty-three studies reported data on methods of reconstruction following NSM. Forty-seven percent of reconstructions following NSMs were two-stage procedures (expander to implant), 41% were one-stage (direct-to-implant) and 12% were autologous reconstructions.
Fifty-five out of 58 included studies in the NSM group described the mastectomy incision used.
Fifteen different incisions were described. In 36 studies (64.3%) more than one type of incision was performed. The various incisions were classified in five categories: the most common incision types were radial, followed by periareolar/circumareolar, inframammary, inverted-T and trans-areolar. Trans-areolar approaches resulted in the highest rate of nipple necrosis. LR in relation to incision location was not reported in any study.
Stolier and colleague performed 82 NSMs without NAC necrosis using a six-o’clock radial incision or a lateral incision if excising a biopsy or breast conserving therapy (BCT) scar (32). The authors also stressed the importance of lighting, use of headlamps, blended current cautery used only for pinpoint hemostasis and the utility of bipolar dissecting scissors.
Other authors also preferred radial or lateral incisions, noting that medial incisions could compromise blood flow (8,29). Paepke and colleague (11) reported only a 1% NAC loss with a periareolar incision, while Regolo and colleague (30) reported a 60% NAC loss with periareolar incision.
Skin-sparing mastectomy (SSM)
Oncological safety
SSM leaves behind more tissue than NSSM. The surgeon leaves superior and inferior skin flaps to preserve the natural skin envelope, removing as much breast tissue as possible, carrying out a dissection above the superficial fascia, leaving in situ only epidermis, dermis and a small amount of subcutaneous fat. Obviously the procedure is more technically demanding when compared to NSSM. Some reports investigating the histological characteristics of skin flaps specimens doubt the oncological safety and equivalence of SSM with NSSM regarding local control of the disease (70,80).
Some authors analyzed skin flap specimens after SSM looking at the amount of residual breast tissue and they found 59.5% of specimens containing residual breast tissue and 9.5% of skin flaps with residual disease, concluding that skin flaps thicker than 5 mm were associated with the presence of residual disease (81,82). Other authors found 23% of skin flaps after SSM involved by residual tumor, in particular at the level of the skin projection of the tumor (83).
Although several studies did not show any statistical difference between NSSM and SSM in terms of LR, other authors showed SSM as an independent predictor of close or positive margins (81-84).
No randomized controlled clinical trials comparing SSM with NSSM have been conducted, but several retrospective series and some prospective cohorts over the past two decades presented data demonstrating the equivalence of SSM and NSSM in terms of LR (71-74,85-88).
The LR rates after NSSM in tumors up to 4 cm was shown to be 10% after 20 years of follow-up (1,2) and our review of the literature found LR rates following SSM to range from 0% to 7% (75-79).
As expected, LR rates after SSM were lower for smaller and low stage tumors with less aggressive characteristics. LR rates after NSSM for DCIS in most series range between 1% and 3% (89-92) and similarly Slavin and colleague showed no recurrences at a follow-up of 45 months after SSM for DCIS (77). Carlson and colleague also presented only one LR after 65 months of follow-up following SSM for DCIS (85) (Table 2).
Newman and colleague presented a 6.2% recurrence rate at a mean follow-up time of 26 months after SSM for T1 and T2 tumors (70). These findings are in line with those of Kroll and Khoo who reported a 7% LR rate at a mean follow-up time of 6 years after SSM (74). Carlson and colleague studied 539 patients undergoing SSM with a mean follow-up time of 65 months and found tumor size, nodal status and lymphovascular invasion to be significant predictors of recurrence, with LR rates of 3%, 10% and 11% for T1, T2 and T3 tumors respectively (85).
Other authors also reported that tumor size, stage, lymph node involvement and poor tumor differentiation were risk factors for LR, showing a LR rate after SSM at a median follow-up of 73 months of 4.5% (83). Spiegel and Butler reported a 5.6% LR rate at 9.8 years in 117 patients treated with SSM (72).
Some authors investigated the use of SSM in small populations of high-risk patients (stage IIB and III) showing promising results, with recurrence rates ranging between 2.6% and 4.6% (73,78,79).
Nipple-sparing mastectomy (NSM)
Oncological safety
Incidence of occult involvement of the nipple by tumor
Many studies reported data on the pathological involvement of the nipple, with the incidence ranging from 0% to 58% (93-109). Excluding small series (less than 100 patients) the range narrows down to 5.6% to 31%.
Obviously patient selection, definition of nipple involvement and pathological methods affect the reported incidence. Many historical studies only included women with small-volume disease. Moreover mastectomy has today become a common procedure for extensive DCIS, while older series excluded DCIS.
Three landmark studies investigating the incidence of microscopic tumor involvement in the NAC presented conflicting results.
Laronga and colleague (105) in 1999 reported that 5.6% of NAC in SSM specimens were positive for occult tumor involvement, concluding that NAC involvement was not an indicator of increased LR or breast cancer specific survival. They reported that central tumor location, multicentricity and positive lymph nodes determine an increased risk of NAC involvement.
In 2001, Cense and colleague (110) reported that up to 58% of mastectomy specimens presented NAC involvement, correlating tumor size, distance from the NAC (<4-5 cm) and positive lymph nodes. They discouraged the use of NSM, recommending patients to undergo BCT, with the benefit of additional radiotherapy.
In 2002 Simmons and colleague (106) studied NAC involvement from mastectomy specimens, finding only 0.9%.
Local recurrence (LR)
No randomized controlled clinical trials comparing NSM versus NSSM or BCT have been found in literature. Evidence deriving from retrospective series and prospective cohorts showed a LR rate after NSM ranging between 0% and 24.1% with high heterogeneity in inclusion criteria, surgical technique and follow-up times.
Benediktsson and colleague (28) performed NSM in patients who were poor BCT candidates, including patients with large and multicentric tumors. They reported a LR rate of 20.8% at a mean follow-up time of 13 years. Despite high LR rates, they reported 0% recurrences at the NAC. They found a statistically significant reduction in the LR rate of 8.5% when adding post-mastectomy radiotherapy (PMRT) to NSM.
Petit et al. (14) and Sookhan et al. (31) reported 0% of NAC LR at short follow-up periods respectively 19 and 10.8 months, thanks to the use of preoperative breast magnetic resonance imaging.
In 2009, Gerber et al. provided (38) data at a follow-up of 10 years, finding only one NAC recurrence out of 112 NSMs performed, without statistical significance in overall LR between NSM and MRM.
In 2012, Petit et al. (111) reported 10% of NAC specimens to be positive after frozen section, but a long-term recurrence rate of 1.18% thanks to the use of intraoperative radiotherapy.
Postoperative complications
NAC necrosis
Nipple-areolar complex necrosis (either partial or complete) was reported in 42 studies (Table 1). The reported rates of NAC necrosis (either partial or complete) ranged from 0% to 60%.
Mastectomy skin flap necrosis
The definition of skin flap necrosis was very variable, with some studies only reporting cases requiring re-interventions and other including all cases of partial or full-thickness necrosis.
Patient satisfaction
Nahabedian and Tsangaris (25) reported good or excellent satisfaction with 11 of 14 reconstructed breasts following NSM. Yueh et al. (41) reported that six out of nine patients were satisfied. The limit of these series is that they did not compare patient satisfaction with patients without NAC preservation.
Gerber and colleague (38) presented the evaluation of aesthetic results of SSM versus NSM after 12 months assessed by patients and surgeons. Patients rated satisfaction with SSM and NSM similarly, with the majority defining the aesthetic outcome as good or excellent. The surgeons rated 74% of NSM as excellent and 26% as good, while rating only 59% of SSM excellent, 22% good and 20% fair (P=0.001).
Didier and colleague (35) studied patient satisfaction with body image, sexuality, cosmetic results and psychological adjustment in two cohorts of patients who underwent NSM and SSM. They did not find any difference in feelings of sexuality, but patients who underwent NSM were more willing to see themselves or be seen naked and had significantly lower ratings for feelings of mutilation. Patients who underwent NSM as compared to SSM reported significantly greater satisfaction with cosmetic results.
Discussion
Despite being commonly offered as an alternative to NSSM, indications for NSM have typically been identical to those for BCT (9,50,112).
Even if no high level evidence is available in literature, NSM has been considered safe in women with small, peripherally located tumors, without multicentricity or risk-reducing mastectomy (50).
While there is data supporting the safety of SSM for larger tumors and more advanced stages, there is less applied to NSM and additional studies, preferably RCTs comparing NSM with NSSM, should be performed.
Schecter and colleague developed (113) an image based model using mammography that helps providing a NAC involvement score (NACIS) based on tumor-nipple distance, pathologic stage and tumor size with 92% sensitivity, 77% specificity and 93% negative predictive value.
Breast MRI can also be considered a useful tool to determine nipple and retroareolar morphology prior to consideration of NSM.
Friedman and colleague (114) correlated preoperative MRI appearance of the nipple in 35 patients with breast cancer undergoing mastectomy with histological results and predicted NAC involvement with 99.5% sensitivity and 100% specificity. They concluded that breast MRI could not only identify retro areolar tumors with or without nipple involvement but also differentiate normal from abnormal nipple.
The literature regarding margins for NSM deeply focuses on the margin at the NAC, but the surgeon should always remember that superficial and deep margins apply too, and this has not been sufficiently studied.
Preoperative counseling for all patients potentially eligible for a NSM is fundamental, discussing potential risks of NAC recurrence but also partial or total NAC necrosis and loss of nipple sensation. Moreover, in case of an intraoperative positive frozen section or complication, patient consent to remove the NAC is mandatory.
RCTs are needed to address almost all questions regarding NSM. However the actual best available evidence deriving from level of evidence III and IV studies provide some characteristics of the patients who can be a candidate for NSM.
The optimal tumor-to-nipple distance has not been defined yet and various prediction models to aid in selection of patients for NSM using preoperative tumor-to-nipple distance values have been proposed; however the total number of mastectomies analyzed in these studies is small and requires validation with larger studies (34,44,109,113).
Although no consensus regarding the oncologic selection criteria exist, general trends include tumor size up to 3 cm and tumor-to-nipple distance greater or equal to 2 cm (28,46,55).
There is no clear consensus regarding whether clinically negative axillary nodes should be required as a selection criteria for NSM, even though axillary nodal status has not been found to influence nipple involvement (14,36,55).
Some authors consider preoperative irradiation as a contraindication for NSM, but no studies validated this assumption (29,66). Several studies included patients who underwent radiation therapy before or after a NSM and reconstruction. Nipple necrosis varied among those studies, ranging from 0% to 54.5%. No meta-analysis could be performed due to the high level of heterogeneity between the studies in terms of irradiation protocols and timing of the treatment.
NSM is not recommended in patients with extensive lymphovascular invasion, estrogen/progesterone receptor-negative tumors and inflammatory carcinomas (33,36).
Risk-reducing NSM may be considered in anatomically appropriate patients (23,36,55).
According to these selection criteria, NSM could be considered an oncologically safe procedure. Because of variable inclusion criteria among included studies, we are not able to assess which selection criteria are more important for overall outcomes.
Numerous incision types have been reported in order to ease the mastectomy and the reconstruction, to preserve the NAC blood flow and to obtain good aesthetic results.
However, there is no one ideal incision choice. However, according to the data presented in the included studies we can conclude that higher rates of NAC necrosis are reported with periareolar/circumareolar patterns and mostly with the transareolar approach (36,43).
NSM can be performed in association with immediate one-stage or two-stage reconstruction.
The direct-to-implant technique decreases costs and seems to lower complication rates, while the two-stage technique allows to improve symmetry, to better define the inframammary fold and optimize the perfusion of the mastectomy skin flaps (34,46).
The incidence of NAC necrosis slightly increases with one-stage reconstruction but the overall complication rate is higher with the two-stage technique.
NSM has been reported also in association with autologous reconstruction [free and pedicled transverse abdominis musculocutaneous (TRAM), deep inferior epigastric perforator (DIEP), superficial inferior epigastric artery (SIEA), latissimus dorsi (LD) and transverse upper gracilis (TUG) flap] (19,44,57,61,65), but due to the high level of heterogeneity between studies and limited patient numbers, it was not possible to draw any conclusion about autologous flaps and their relation to NAC and LR.
In the majority of the included studies, subareolar tissue was sent as a frozen section or as a permanent pathologic specimen or both, with a high level of heterogeneity among studies.
The sensitivity and specificity of frozen section subareolar biopsy for occult malignancy has been shown to be 91% and 98%, respectively (28). Some surgeons however send subareolar tissue for permanent section only and in these cases the NAC can be resected at the second stage of reconstruction.
However the rate of occult carcinoma within the NAC (most often DCIS) has been shown to be low, ranging from 1.2% to 5.9% (55).
There is no consensus regarding intraoperative or delayed radiation therapy on the NAC.
Reported LR rates after NSM vary very widely across studies (from 0% to 24.1%). The high level of heterogeneity among studies may be attributed to several factors, including the variability and inadequacy of follow-up length (10 months to 15 years), the variability in the tumor stage considered and the variability in additional treatments.
This review presents the great limitation of including only retrospective series and some prospective cohorts, having high heterogeneity in the characteristics of included patients, additional treatments received, surgical technique and reported methods of outcome.
NSM is generally considered oncologically safe in selected patients, but the decision to proceed with a NSM should always take into account oncological and anatomical selection criteria with the selection of the most appropriate skin incision and the best reconstructive option, always performing accurate subareolar tissue sampling (115-118).
The level of the evidence behind conservative mastectomies appears to be low and RCTs comparing BCT and MRM with skin-sparing techniques would be advisable in order to obtain higher levels of evidence on oncological and reconstructive outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Veronesi U, Cascinelli N, Marubini E, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [PubMed]
- Madden JL. Modified radical mastectomy. Surg Gynec Obstet 1965;121:1221-30. [PubMed]
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991;87:1048-53. [PubMed]
- Carlson GW. Skin sparing mastectomy: anatomic and technical considerations. Am Surg 1996;62:151-5. [PubMed]
- Uriburu JL, Vuoto HD, Cogorno L, et al. Local recurrence of breast cancer after skin-sparing mastectomy following core needle biopsy: case reports and review of the literature. Breast J 2006;12:194-8. [PubMed]
- Sacchini V, Pinnotti JA, Banos AC, et al. Nipple sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [PubMed]
- Chung AP, Sacchini V. Nipple-sparing mastectomy: where are we now? Surg Oncol 2008;17:261-6. [PubMed]
- Folli S, Curcio A, Buggi F, et al. Improved sub-areolar breast tissue removal in nipple-sparing mastectomy using hydrodissection. Breast 2012;21:190-3. [PubMed]
- Paepke S, Schmid R, Fleckner S, et al. Subcutaneous mastectomy with conservation of the nipple-areola skin: broadening the indications. Ann Surg 2009;250:288-92. [PubMed]
- Niemeyer M, Paepke S, Schmid R, et al. Extended indications for nipple-sparing mastectomy. Breast J 2011;17:296-9. [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45; discussion 45. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: One thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [PubMed]
- Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol 2013;20:1436-43. [PubMed]
- Verheyden CN. Nipple-sparing total mastectomy of large breasts: The role of tissue expansion. Plast Reconstr Surg 1998;101:1494-1500; discussion 1501. [PubMed]
- Sufi PA, Gittos M, Collier DS. Envelope mastectomy with immediate reconstruction (EMIR). Eur J Surg Oncol 2000;26:367-70. [PubMed]
- Mustonen P, Lepistö J, Papp A, et al. The surgical and onco-logical safety of immediate breast reconstruction. Eur J Surg Oncol 2004;30:817-23. [PubMed]
- Dao TN, Verheyden CN. TRAM flaps: A reconstructive option after bilateral nipple-sparing total mastectomy. Plast Reconstr Surg 2005;116:986-92. [PubMed]
- Margulies AG, Hochberg J, Kepple J, et al. Total skin-sparing mastectomy without preservation of the nipple-areola complex. Am J Surg 2005;190:907-12. [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: Rationale and technique. Breast J 2005;11:173-8. [PubMed]
- Bistoni G, Rulli A, Izzo L, et al. Nipple-sparing mastectomy. Preliminary results. J Exp Clin Cancer Res 2006;25:495-7. [PubMed]
- Caruso F, Ferrara M, Castiglione G, et al. Nipple sparing subcutaneous mastectomy: Sixty-six months follow-up. Eur J Surg Oncol 2006;32:937-40. [PubMed]
- Komorowski AL, Zanini V, Regolo L, et al. Necrotic complications after nipple- and areola- sparing mastectomy. World J Surg 2006;30:1410-3. [PubMed]
- Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg 2006;117:1083-90. [PubMed]
- Denewer A, Farouk O. Can nipple-sparing mastectomy and immediate breast reconstruction with modified extended latissimus dorsi muscular flap improve the cosmetic and functional outcome among patients with breast carcinoma? World J Surg 2007;31:1169-77. [PubMed]
- Mosahebi A, Ramakrishnan V, Gittos M, et al. Aesthetic outcome of different techniques of reconstruction following nipple-areola-preserving envelope mastectomy with immediate reconstruction. Plast Reconstr Surg 2007;119:796-803. [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: A prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [PubMed]
- Crowe JP, Patrick RJ, Yetman RJ, et al. Nipple-sparing mastectomy update: One hundred forty-nine procedures and clinical outcomes. Arch Surg 2008;143:1106-10; discussion 1110. [PubMed]
- Regolo L, Ballardini B, Gallarotti E, et al. Nipple sparing mastectomy: An innovative skin incision for an alternative approach. Breast 2008;17:8-11. [PubMed]
- Sookhan N, Boughey JC, Walsh MF, et al. Nipple-sparing mastectomy: Initial experience at a tertiary center. Am J Surg 2008;196:575-7. [PubMed]
- Stolier AJ, Sullivan SK, Dellacroce FJ. Technical considerations in nipple-sparing mastectomy: 82 consecutive cases without necrosis. Ann Surg Oncol 2008;15:1341-7. [PubMed]
- Voltura AM, Tsangaris TN, Rosson GD, et al. Nipple-sparing mastectomy: Critical assessment of 51 procedures and implications for selection criteria. Ann Surg Oncol 2008;15:3396-401. [PubMed]
- Chen CM, Disa JJ, Sacchini V, et al. Nipple-sparing mastectomy and immediate tissue expander/implant breast recon-struction. Plast Reconstr Surg 2009;124:1772-80. [PubMed]
- Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 2009;118:623-33. [PubMed]
- Garcia-Etienne CA, Cody Iii HS. Nipple-sparing mastectomy: Initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J 2009;15:440-9. [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: An extended follow-up study. Ann Surg 2009;249:461-8. [PubMed]
- Munhoz AM, Aldrighi C, Montag E, et al. Optimizing the nipple-areola sparing mastectomy with double concentric periareolar incision and biodimensional expander-implant reconstruction: aesthetic and technical refinements. Breast 2009;18:356-67. [PubMed]
- Sakamoto N, Fukuma E, Higa K, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol 2009;16:3406-13. [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: Evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [PubMed]
- Babiera G, Simmons R. Nipple-areolar complex-sparing mastectomy: feasibility, patient selection and technique. Ann Surg Oncol 2010;17:245-8. [PubMed]
- Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: Optimizing mastectomy and reconstruction. Ann Plast Surg 2010;65:140-3. [PubMed]
- Kim HJ, Park EH, Lim WS, et al. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: A single center study. Ann Surg 2010;251:493-8. [PubMed]
- Luo D, Ha J, Latham B, et al. The accuracy of intraoperative subareolar frozen section in nipple-sparing mastectomies. Ochsner J 2010;10:188-92. [PubMed]
- Radovanovic Z, Radovanovic D, Golubovic A, et al. Early complications after nipple- sparing mastectomy and immediate breast reconstruction with silicone prosthesis: Results of 214 procedures. Scand J Surg 2010;99:115-8. [PubMed]
- Rusby JE, Gui GP. Nipple-sparing mastectomy in women with large or ptotic breasts. J Plast Reconstr Aesthet Surg 2010;63:e754-5. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: Cosmetic outcomes and technical refinements. Plast Reconstr Surg 2010;126:1460-71. [PubMed]
- Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg 2011;212:686-93; discussion 693. [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: The Memorial Sloan- Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22. [PubMed]
- Harness JK, Vetter TS, Salibian AH. Areola and nipple-areola-sparing mastectomy for breast cancer treatment and risk reduction: Report of an initial experience in a community hospital setting. Ann Surg Oncol 2011;18:917-22. [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [PubMed]
- Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: oncological safety and incision selection. Aesthet Surg J 2011;31:310-9. [PubMed]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg 2011;19:129-33. [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [PubMed]
- Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: Can we predict the factors predisposing to necrosis? Eur J Surg Oncol 2012;38:125-9. [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: A powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol 2012;19:3171-6. [PubMed]
- Kneubil MC, Lohsiriwat V, Curigliano G, et al. Risk of locoregional recurrence in patients with false-negative frozen section or close margins of retroareolar specimen in nipple- sparing mastectomy. Ann Surg Oncol 2012;19:4117-23. [PubMed]
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: Technical aspects and aesthetic outcomes. Ann Plast Surg 2012;68:446-50. [PubMed]
- Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: Results of a prospective practice improvement study. Plast Reconstr Surg 2012;129:901e-908e. [PubMed]
- Schneider LF, Chen CM, Stolier AJ, et al. Nipple-sparing mastectomy and immediate free-flap reconstruction in the large ptotic breast. Ann Plast Surg 2012;69:425-8. [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [PubMed]
- Yang SJ, Eom JS, Lee TJ, et al. Recipient vessel selection in immediate breast reconstruction with free abdominal tissue transfer after nipple-sparing mastectomy. Arch Plast Surg 2012;39:216-21. [PubMed]
- Blechman KM, Karp NS, Levovitz C, et al. The lateral inframammary fold incision for nipple-sparing mastectomy: Outcomes from over 50 immediate implant-based breast reconstructions. Breast J 2013;19:31-40. [PubMed]
- Lohsiriwat V, Rotmensz N, Botteri E, et al. Do clinicopathological features of the cancer patient relate with nipple areolar complex necrosis in nipple-sparing mastectomy? Ann Surg Oncol 2013;20:990-6. [PubMed]
- Tanna N, Broer PN, Weichman KE, et al. Microsurgical breast reconstruction for nipple-sparing mastectomy. Plast Reconstr Surg 2013;131:139e-147e. [PubMed]
- Sahin I, Isik S, Alhan D, et al. One-staged silicone implant breast reconstruction following bilateral nipple-sparing prophylactic mastectomy in patients at high risk for breast cancer. Aesthetic Plast Surg 2013;37:303-11. [PubMed]
- Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 1998;5:620-6. [PubMed]
- Carlson GW, Page A, Johnson E, et al. Local recurrence of ductal carcinoma in situ after skin-sparing mastectomy. J Am Coll Surg 2007;204:1074-8. [PubMed]
- Spiegel AJ, Butler CE. Recurrence following treatment of ductal carcinoma in situ with skin-sparing mastectomy and immediate breast reconstruction. Plast Reconstr Surg 2003;111:706-11. [PubMed]
- Lim W, Ko BS, Kim HJ, et al. Oncological safety of skin sparing mastectomy followed by immediate reconstruction for locally advanced breast cancer. J Surg Oncol 2010;102:39-42. [PubMed]
- Kroll SS, Khoo A. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg 1999;104:421-5. [PubMed]
- Greenway RM, Schlossberg L, Dooley WC. Fifteen-year series of skin-sparing mastectomy for stage 0 to 2 breast cancer. Am J Surg 2005;190:918-22. [PubMed]
- Rubio IT, Mirza N, Sahin AA, et al. Role of specimen radiography in patients treated with skin-sparing mastectomy for ductal carcinoma in situ of the breast. Ann Surg Oncol 2000;7:544-8. [PubMed]
- Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg 1998;102:49-62. [PubMed]
- Foster RD, Esserman LJ, Anthony JP, et al. Skin-sparing mastectomy and immediate breast reconstruction: a prospective cohort study for the treatment of advanced stages of breast carcinoma. Ann Surg Oncol 2002;9:462-6. [PubMed]
- Downes KJ, Glatt BS, Kanchwala SK, et al. Skin-sparing mastectomy and immediate reconstruction is an acceptable treatment option for patients with high-risk breast carcinoma. Cancer 2005;103:906-13. [PubMed]
- Sotheran WJ, Rainsbury RM. Skin-sparing mastectomy in the UK - A review of current practice. Ann R Coll Surg Engl 2004;86:82-6. [PubMed]
- Torresan RZ, DosSantos CC, Brenelli H, et al. Residual glandular tissue after skin-sparing mastectomies. Breast J 2005;11:374-5. [PubMed]
- Torresan RZ, DosSantos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [PubMed]
- Ho CM, Mak CK, Lau Y, et al. Skin involvement in invasive breast carcinoma: safety of skin-sparing mastectomy. Ann Surg Oncol 2003;10:102-7. [PubMed]
- Lovrics PJ, Cornacchi SD, Farrokhyar F, et al. The relationship between surgical factors and margin status after breast-conservation surgery for early stage breast cancer. Am J Surg 2009;197:740-6. [PubMed]
- Carlson GW, Styblo TM, Lyles RH, et al. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol 2003;10:108-12. [PubMed]
- Langstein HN, Cheng MH, Singletary SE, et al. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg 2003;111:712-20. [PubMed]
- Medina-Franco H, Vasconez LO, Fix RJ, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg 2002;235:814-9. [PubMed]
- Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol 1999;6:676-81. [PubMed]
- Al Mushawah F, Rastelli A, Pluard T, et al. Metastatic invasive breast cancer recurrence following curative-intent therapy for ductal carcinoma in situ. J Surg Res 2012;173:10-5. [PubMed]
- Godat LN, Horton JK, Shen P, et al. Recurrence after mastectomy for ductal carcinoma in situ. Am Surg 2009;75:592-5. [PubMed]
- Kelley L, Silverstein M, Guerra L. Analyzing the risk of recurrence after mastectomy for DCIS: a new use for the USC/Van nuys prognostic index. Ann Surg Oncol 2011;18:459-62. [PubMed]
- Kim JH, Tavassoli F, Haffty BG. Chest wall relapse after mastectomy for ductal carcinoma in situ: a report of 10 cases with a review of the literature. Cancer J 2006;12:92-101. [PubMed]
- Smith J, Payne WS, Carney JA. Involvement of the nipple and areola in carcinoma of the breast. Surg Gynecol Obstet 1976;143:546-8. [PubMed]
- Parry RG, Cochran TC Jr, Wolfort FG. When is there nipple involvement in carcinoma of the breast? Plast Reconstr Surg 1977;59:535-7. [PubMed]
- Lagios MD, Gates EA, Westdahl PR, et al. A guide to the frequency of nipple involvement in breast cancer. A study of 149 consecutive mastectomies using a serial subgross and correlated radiographic technique. Am J Surg 1979;138:135-42. [PubMed]
- Wertheim U, Ozzello L. Neoplastic involvement of nipple and skin flap in carcinoma of the breast. Am J Surg Pathol 1980;4:543-9. [PubMed]
- Andersen JA, Gram JB, Pallesen RM. Involvement of the nipple and areola in breast cancer. Value of clinical findings. Scand J Plast Reconstr Surg 1981;15:39-42. [PubMed]
- Quinn RH, Barlow JF. Involvement of the nipple and areola by carcinoma of the breast. Arch Surg 1981;116:1139-40. [PubMed]
- Morimoto T, Komaki K, Inui K, et al. Involvement of nipple and areola in early breast cancer. Cancer 1985;55:2459-63. [PubMed]
- Kissin MW, Kark AE. Nipple preservation during mastectomy. Br J Surg 1987;74:58-61. [PubMed]
- Menon RS, van Geel AN. Cancer of the breast with nipple involvement. Br J Cancer 1989;59:81-4. [PubMed]
- Santini D, Taffurelli M, Gelli MC, et al. Neoplastic involvement of nipple–areolar complex in invasive breast cancer. Am J Surg 1989;158:399-403. [PubMed]
- Suehiro S, Inai K, Tokuoka S, et al. Involvement of the nipple in early carcinoma of the breast. Surg Gynecol Obstet 1989;168:244-8. [PubMed]
- Vyas JJ, Chinoy RF, Vaidya JS. Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol 1998;24:15-6. [PubMed]
- Laronga C, Kemp B, Johnston D, et al. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol 1999;6:609-13. [PubMed]
- Simmons RM, Brennan M, Christos P, et al. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol 2002;9:165-8. [PubMed]
- Afifi RY, El-Hindawy A. Analysis of nipple–areolar complex involvement with mastectomy: can the nipple be preserved in Egyptian patients receiving skin-sparing mastectomy? Breast J 2004;10:543-5. [PubMed]
- Vlajcic Z, Zic R, Stanec S, et al. Nipple–areola complex preservation: predictive factors of neoplastic nipple–areola complex invasion. Ann Plast Surg 2005;55:240-4. [PubMed]
- Rusby JE, Brachtel EF, Othus M, et al. Development and validation of a model predictive of occult nipple involvement in women undergoing mastectomy. Br J Surg 2008;95:1356-61. [PubMed]
- Cense HA, Rutgers EJ, Lopes Cardozo M, et al. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol 2001;27:521-6. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012;23:2053-8. [PubMed]
- Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg 2004;188:78-84. [PubMed]
- Schecter AK, Freeman MB, Giri D, et al. Applicability of the nipple-areola complex-sparing mastectomy: A prediction model using mammography to estimate risk of nipple-areola complex involvement in breast cancer patients. Ann Plast Surg 2006;56:498-504; discussion 504. [PubMed]
- Friedman EP, Hall-Craggs MA, Mumtaz H, et al. Breast MR and the appearance of the normal and abnormal nipple. Clin Radiol 1997;52:854-61. [PubMed]
- Nava MB, Catanuto G, Pennati A, et al. Conservative mastectomies. Aesthetic Plast Surg 2009;33:681-6. [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10. [PubMed]
- della Rovere GQ, Nava MB, Bonomi R, et al. Skin-reducing mastectomy with breast reconstruction and sub-pectoral implants. J Plast Reconstr Aesthet Surg 2008;61:1303-8. [PubMed]
- Nava MB, Ottolenghi J, Pennati A, et al. Skin/nipple sparing mastectomies and implant-based breast reconstruction in patients with large and ptotic breast: oncological and reconstructive results. Breast 2012;21:267-71. [PubMed]