Fat grafting and breast reconstruction: tips for ensuring predictability
Introduction
Autologous fat grafting is widely used in breast surgery to refine and optimize aesthetic outcomes, both in breast reconstruction as well as in breast aesthetic surgery. In breast reconstructive surgery, it is primarily used as an adjunct to standard breast reconstruction procedures (1) although the feasibility of using fat alone as a primary method of reconstruction has also been recently demonstrated (2). As an adjunct to reconstruction, fat grafting has been successfully used for a variety of indications including the correction of volume, shape, and contour deformities (3-9); treatment of irradiated breast tissue; and priming of the irradiated field for breast reconstruction (10-12). There is some evidence that fat grafting may also help in mitigating postmastectomy pain syndrome (13) and in the treatment of capsular contracture (10).
Fat is an appealing filler material for it is biocompatible, is abundantly available, can be easily harvested and processed, and can be injected in controlled amounts. Despite the appeal of fat and widespread adoption of fat grafting in plastic and reconstructive surgery, challenges/concerns remain with this procedure. In particular, obtaining predictable, reliable, and consistent outcomes remains a significant challenge and is due to the high variability in graft volume retention. As much as 40-60% of the volume of fat injected could be lost (4,14-16) due to necrosis or resorption (17). The unpredictable outcome is largely attributed to the technique of fat grafting that encompasses three stages: procurement, processing, and placement of the fat.
At present, there is no published consensus on the optimal technique for fat grafting. The purpose of this article is to review current techniques at each stage of fat grafting and provide tips on best practices based on the published literature as well as our extensive clinical experience.
Procurement
Donor site
Fat can be harvested from a number of sites, including the abdomen; medial, lateral, or anterior thighs; trochanteric region; flank; lower back, and knees. Whether there is an optimal donor site for fat grafting remains to be established but evidence suggests that some sites may be preferable to others.
In an in vitro study, Padoin et al. showed that fat from the lower abdomen and medial thighs consist of a higher concentration of adipose-derived stem cells compared with fat from the upper abdomen, trochanteric region, knee, and flank (18). As adipose-derived stem cells are believed to play a vital role in graft survival through adipogenesis and angiogenesis (19-22), these data suggest that the lower abdomen and medial thighs may be preferred over other donor sties.
Other studies, however, have reported no influence of the donor site on fat viability (23-26). In a study by Rohrich et al., fat removed from the abdomen, thigh, flank, or knee were immediately evaluated without treatment for adipocyte viability as well as after centrifugation as a method of processing. No statistically significant difference in fat viability was seen among the donor sites in both untreated and treated samples in this in vitro study (23). In an in vivo study, Ullmann et al. harvested fat from the abdomen, lateral thigh, and breast of a single patient and grafted it into nude mice. They found no significant difference in fat graft take across the different harvest sites (24). In another in vivo study, Li et al. harvested fat from the flank, upper abdomen, lower abdomen, inner thigh, and lateral thigh of six young female patients. Again no significant differences were found on fat graft take in nude mice (25). In a clinical study, Small et al. harvested fat from the abdomen and thighs of 73 patients that was used in fat grafting to their reconstructed breasts; 46 patients (66 breasts) received fat from the abdomen and 27 patients (43 breasts) received fat from the thighs. Fat volume retention evaluated at various time points (16, 49, and 140 days) after grafting using 3-dimensional scanning showed no significant difference between fat harvested from the abdomen and the thighs (26). These studies, however, did not take into consideration patient characteristics that might influence graft survival.
Geissler et al. suggested that patients’ age might influence adipocyte survival and that age should be taken into consideration when selecting a donor site (27). They found that in younger patients (≤45 years) adipocyte viability was greater in the lower abdomen than in the flank. In older patients (≥46 years), there was no difference in viability of adipocytes from the lower abdomen and the flank; but, compared with younger patients, viability of fat from the flank region was greater in older patients. There was no difference in inner thigh fat viability between the two age groups. The body mass index (BMI) of the patients [normal (BMI <25) or overweight (BMI ≥25)] did not appear to influence viability of fat from any particular donor site.
Collectively, these data suggest that the lower abdomen and medial thighs may be preferred over other donor sites both from the standpoint of adipose-derived stem cells and the age of patients. However, oftentimes, availability of fat may dictate the site chosen, especially in thin patients. Also, some patients may have a preference for a specific donor site.
Infiltration
Prior to fat aspiration, the donor site is typically infused with tumescent solution, usually consisting of a local anesthetic (lidocaine, ropivacaine, prilocaine, or bupivacaine) for pain relief and epinephrine for hemostasis in Lactated Ringer’s solution or normal saline.
Several studies have examined the effect of the local anesthetic or epinephrine on fat viability. In an in vitro study, cell attachment in culture, cell morphology, proliferation, or adipocyte metabolic activity appeared to be unaffected by the use of lidocaine and epinephrine (28). Moreover, various doses of epinephrine (1:100,000, 1:200,000, and 1:400,000) did not impact fat cell viability (29). When the procured fat was implanted in nude mice, local anesthesia solution consisting of lidocaine and epinephrine administered to the fat donor site was found not to alter the take of fat grafts or had any influence on adipocyte viability (30). However, there is some evidence that local anesthetics may modulate the viability of isolated preadipocytes (lidocaine, ropivacaine, and prilocaine, but not bupivacaine) as well as their differentiation into mature adipocytes in in vitro studies (31,32). But, the preadipocytes in these studies were directly exposed to high concentrations of anesthetics (2%) compared with in vivo conditions where the relative concentration of anesthetic would be lower due to dilution effects.
In summary, there is no strong evidence to suggest that the use of local anesthetics or epinephrine adversely affects fat graft survival.
Mechanism of liposuction
Fat can be harvested using a number of techniques, including conventional liposuction (syringe with vacuum suction), power-assisted liposuction (specialized cannula with mechanized movement), hand-held syringe liposuction (syringe with manual suction, Coleman technique), internal ultrasound-assisted liposuction (specialized cannula that transmits ultrasound vibrations within the body), and external ultrasound-assisted liposuction (ultrasonic energy applied from outside the body, through the skin). In addition, there are also fat harvesting devices such as the Viafill system (Lipose Corp., Maitland, Fla.; manual syringe liposuction) and LipiVage system (Genesis Biosystems, Lewisville, Texas; syringe aspiration at low vacuum pressure). The influence of liposuction techniques on fat viability and retention has been evaluated in a number of studies, most of which have compared conventional liposuction with suction- or power-assisted liposuction.
Leong et al. compared syringe liposuction to pump-assisted liposuction and found no differences in cell viability, cell metabolic activity, or adipogenic responses of cultured mesenchymal precursor cells processed from pump and syringe lipoaspirates (33). In contrast, Pu et al. demonstrated that syringe liposuction (Coleman technique) yields a greater number of viable adipocytes and sustains a more optimal level of cellular function within fat grafts than conventional liposuction, although normal histologic structure was maintained in fat grafts obtained by both methods (34). Similarly, the newer harvesting devices, LipiVage (Genesis Biosystems) and Viafill (Lipose Corp.), also fared better than conventional liposuction. Liposuction using the LipiVage (Genesis Biosystems) or Viafill (Lipose Corp.) systems was associated with higher yields of viable adipocytes, demonstrating the importance of low pressure suction for fat viability (35,36). Gonzalez et al. have proposed the use of fine needle aspiration (comprising a 2-mm blunt needle with a 10-cc syringe adapted to a fine-needle aspiration apparatus) as an alternate method of liposuction which exerts a significantly lower pressure than hand-held syringe liposuction (using a 3-mm liposuction cannula with a 60-cc syringe). Fine needle aspiration yielded better fat viability (37).
Two studies evaluated the impact of ultrasound-assisted liposuction on adipose viability. In one study, Rohrich et al. showed that external ultrasound had no significant impact on adipocyte cellular integrity while internal ultrasound resulted in thermal liquefaction of mature adipocytes (38). Similarly, Shiffman and Mirrafati also showed that external ultrasound does not destroy fat cells, although it produces smaller bundles of fat (39). A more recent study that used a third generation internal ultrasound device (VASER; Sound Surgical Technologies, Louisville, CO) found no difference in fat graft retention between the ultrasound device and suction-assisted liposuction in a xenograft model (40).
In addition to the method of liposuction, other variables such as cannula size and suction pressure employed during liposuction could also have an impact on adipocyte viability. Irrespective of the method of liposuction, a blunt-tip harvesting cannula is utilized to withdraw the lipoaspirate from the donor site. Two studies have established that the use of large bore size cannulas yields a greater number of viable adipocytes in the lipoaspirate. In one study, a 4-mm cannula was shown to be better than 2-3 mm cannulas (41) and in another study, a 6-mm cannula was shown to be better than 4- and 2-mm cannulas (42). Shiffman and Mirrafati tested the effect of various suction pressures on adipocyte viability and noted adipocyte damage of greater than 10% with the use of −700 mmHg vacuum (39). This finding was corroborated by Cheriyan et al. who demonstrated that a low harvest pressure (−250 mmHg) resulted in an adipocyte count that was 47% higher than a high harvest pressure (−760 mmHg) (43).
Based on these studies, it appears that currently available liposuction methods are all relatively adipocyte friendly harvesting techniques. When using suction-assisted liposuction, the use of low suction pressure is preferable. Although there is no clear evidence for the superiority of any one type of harvesting technique, a survey of members of the American Society of Plastic Surgeons revealed that hand-held manual suction appears to be the preferred technique (1). With respect to harvesting cannulas, larger sizes (≥4 mm) may be preferable as they appear to increase viable adipocyte yield.
Processing
Prior to fat grafting, the harvested fat is typically processed in some manner to eliminate tumescent fluid, blood, cell fragments, and free oil (from disrupted adipocytes) (17). By eliminating these contaminants, processing aims to retain viable adipocytes in a concentrated form, which is believed to enhance graft take (44,45). The most commonly performed fat processing methods are filtration, centrifugation, or sedimentation (decantation) (1). The filtration technique uses a platform for concentrating fat cells and separating them from fluids, oil, and debris. As a platform, various materials such as filters with defined pore size, cotton gauze, metal sieve, mesh, and operating room cloth have been used for the purpose of filtering lipoaspirate. In the centrifugation technique, the syringe containing the lipoaspirate is placed in a centrifuge and spun at a specified speed and time. In the sedimentation technique, the syringe containing the lipoaspirate is allowed to sit for decantation to occur under the action of gravity. In a variation of this technique the lipoaspirate is washed with 1-3 times the volume with normal saline or Lactated Ringer’s solution and then left to decant under gravity. In all three cases, centrifugation, sedimentation, and washing, the lipoaspirate is separated into three layers: an upper oil layer, a middle purified and concentrated fat layer, and a lower aqueous layer consisting of blood, infiltration or washing liquids. In addition, in the centrifugation technique, a pellet is seen at the bottom of the centrifuge.
Attempts to determine the optimal method of fat processing has thus far been inconclusive because of conflicting results as summarized below.
Centrifugation vs. sedimentation
In a prospective, randomized, double-blind clinical study, Butterwick grafted fat processed by centrifugation in one hand and non-centrifuged fat (sedimentation) in the contralateral hand in 14 patients (46). At 3 and 5 months postoperatively, hands that received centrifuged fat displayed improved aesthetic outcome measured subjectively and objectively (vein prominence and depth of metacarpal space). In direct contrast to this study, Khater et al. demonstrated in a series of 51 patients lipofilling with non-centrifuged fat (sedimentation) that was serum washed resulted in improved clinical outcome at 1-year postoperatively compared with lipofilling with centrifuged fat (3,400 rpm for 3 min) (47). In vitro examination revealed the presence of a greater amount of preadipocytes in the cultured non-centrifuged adipose tissue and more distinctly expressed cell proliferation. Likewise, Condé-Green et al. showed that cell count per high-powered field of intact nucleated adipocytes was significantly greater in decanted lipoaspirates, whereas centrifuged samples showed a greater majority of altered adipocytes (48). However, mesenchymal stem cell concentration was significantly higher in washed lipoaspirates compared to decanted and centrifuged samples but the pellet collected at the bottom of the centrifuged samples had the highest concentration. The authors concluded that washing may be the best processing technique for adipose tissue graft take and recommended that if centrifugation is used, the pellet containing mesenchymal stem cells should be added to the concentrated adipose phase to augment graft take.
Centrifugation vs. filtration
In a clinical study, using subjective and objective evaluations, clinical outcome was deemed comparable between centrifuged fat (3,000 rpm for 3 min) and filtered, washed fat (49). In this prospective, double-blind study in 25 patients undergoing facial fat transplantation, half the face was injected with filtered and washed fat while the other half injected with centrifuged fat. At an average follow-up period of 12 months, the implanted hemifacial regions demonstrated comparable results.
In a nude mouse model, Ramon et al. demonstrated that human fat processed by operating room cloth concentration was comparable to that processed by centrifugation (50). After 16 weeks postimplantation, no significant differences in weight and volume of explanted fat graft were noted between the two processing methods. Histologic analysis of the fat grafts revealed significantly less fibrosis within the graft processed by operating room cloth, suggesting that the quality of the fat graft was better than that processed via centrifugation. Similar to this study, Minn et al. reported no significant differences in fat graft survival rates in nude mice between grafts prepared by centrifugation, metal sieve concentration, and cotton gauze concentration (51). Two recent studies have further corroborated the comparable outcome of centrifugation and filtering as processing methods. Salinas et al. reported that lipoaspirate processed by centrifugation at 1,200 ×g or using mesh/gauze concentration yields an equivalent amount of concentrated fat, about 90% concentrated fat (45). In addition, the number of adipose-derived stem cells in 1 g of concentrated fat was equivalent. The explanted fat grafts from the two methods also exhibited equivalent weights after 4 or 6 weeks implantation in nude mice. Fisher et al. demonstrated in the nude mice model that filtration (using an 800-μm pore size filter) and centrifugation both effectively removed fluid fractions and resulted in comparable graft retention (40). When they compared these two methods with cotton gauze rolling, the latter method resulted in greater fat graft retention. The authors suggested that cotton gauze rolling may be best suited for grafting cosmetically sensitive areas of the body in which optimal retention is critical and lower total graft volumes are needed while filtration and centrifugation would be preferable when larger volumes are required.
Optimal centrifugation conditions
The Coleman technique is the most widely used centrifugation protocol in which the lipoaspirate is centrifuged at ~1,200 ×g (3,000 rpm) for 3 min (52). Some studies that evaluated optimal centrifugation conditions have also suggested a centrifugal force of ~1,200 ×g (3,000 rpm) to be optimal in concordance with Coleman’s protocol. Kurita et al. compared 6 centrifugation speeds (0, 400, 700, 1,200, 3,000, or 4,200 ×g for 3 min) to evaluate the effects of centrifugation on lipoaspirates and graft take in nude mice (53). They reported that centrifugation at more than 3,000 ×g significantly damaged adipose-derived stem cells and recommended 1,200 ×g as an optimized centrifugal force for obtaining good short- and long-term results in adipose transplantation. In an in vitro study, Kim et al. compared the number of viable fat cells after fat samples were centrifuged for 1, 3, and 5 min at 1,500, 3,000, and 5,000 rpm, respectively, with uncentrifuged fat (29). Cell survival rates were significantly lower when centrifuged at 1,500 and 3,000 rpm for more than 5 min and when centrifuged at 5,000 rpm for more than 1 min. The authors recommended centrifugation at 3,000 rpm for 3 min as being optimal.
Other studies have contended that even lower centrifugal forces than the Coleman protocol may be more adipocyte friendly. Hoareau et al. subjected adipose tissue to soft (100 ×g/1 min and 400 ×g/1 min) and strong (900 ×g/3 min and 1,800 ×g/10 min) centrifugal forces and evaluated graft viability in immunodeficient mice (54). Strong centrifugation resulted in 3-fold more adipocyte death than soft centrifugation. The authors suggested that soft centrifugation (400 ×g/1 min) seems to be the most appropriate protocol for the reinjection of adipose tissue.
While other studies have found that centrifugation irrespective of the centrifugal force is deleterious to adipocytes. Xie et al. subjected lipoaspirates to four different centrifugal forces (1,000, 2,000, 3,000, 4,000 rpm) and evaluated fat cell viability via an in vitro glucose transport test (55). Compared with no centrifugation, centrifuged samples demonstrated a significant and linear reduction in fat cell viability with increasing centrifugal force. Histological analyses revealed significantly distorted and fractured adipocytes when the centrifugal force reached 4,000 rpm (1,145 ×g).
Yet other studies suggest that centrifugal force has no effect on adipocyte viability. Using eight different centrifugal forces (up to 20,000 ×g) Pulsfort et al. showed no significant alterations in the viability of centrifuged adipocytes (56). Further, cultivation of isolated adipocyte after centrifugation revealed no apoptotic changes. However, lipoaspirates centrifuged with higher accelerations seemed to be better cleansed of oil and cell debris than samples treated with lower centrifugal forces. Lee et al. centrifuged lipoaspirates at various speeds (50 ×g, 200 ×g, 1,200 ×g, 5,000 ×g, 10,000 ×g, and 23,000 ×g) for 3 min and injected aliquots of purified fat into nude mice to evaluate graft weight and histology at 4 weeks post implantation (57). A statistically significant linear increase in graft take was seen as the speed was increased up to 10,000 ×g but there was no histological difference between the grafts. In a subsequent study by the same group, the increase in graft take with increasing centrifugation speeds was associated with increasing concentration of the adipocyte fraction as the speed was increased to 5,000 ×g (45). Beyond 5,000 ×g the adipocyte fraction did not change significantly, suggesting that 5,000 ×g results in nearly 100 percent concentrated fat. Adding back tumescent solution (surgical or fresh) or cell pellet to the concentrated fat before grafting resulted in reduced graft retention while adding back oil did not affect graft take.
Newer processing techniques
Fat processing using the conventional methods (centrifugation, filtering, and sedimentation) can be cumbersome and time consuming to perform in the operating room, particularly if processing large volumes of fat. In order to simplify fat processing, commercial fat processing systems are now available that simultaneously wash and filter lipoaspirates in a closed system (e.g., PuregraftTM, Cytori Therapeutics, Inc., San Diego, CA and REVOLVETM, LifeCell Corp., Branchburg, NJ). These systems have also been shown to result in greater fat retention than conventional methods. Zhu et al. demonstrated that grafts prepared by the PuregraftTM (Cytori Therapeutics) system exhibited significantly reduced blood cell and free lipid content with significantly greater adipose tissue viability than grafts prepared by sedimentation or Coleman centrifugation (58). Ansorge et al. showed that the REVOLVETM (LifeCell Corp.) system yielded significantly less blood cell debris, a higher percentage of adipose tissue, and a lower percentage of free oil compared with sedimentation or Coleman centrifugation (59). In nude mice, fat tissue retention from REVOLVETM (LifeCell Corp.) samples was significantly higher than that from decanted samples and similar to that from centrifuged samples.
Based on the published literature, any one method of fat processing doesn’t appear to be superior. A survey of American Society of Plastic Surgeons indicates that filtering, sedimentation, and centrifugation are all equally popular (1).
Our current protocol for fat processing is via the REVOLVETM (LifeCell Corp.) system; prior to which, we used the Coleman centrifugation technique. With the REVOLVETM (LifeCell Corp.) system, the lipoaspirate can be channeled directly into the system (Figure 1), which is convenient as this eliminates lipoaspirate handling in syringes. In addition, according to the manufacturer, the system can process up to 800 mL of lipoaspirate in less than 15 min which may translate to a reduction in operating room time. To evaluate this claim, we performed a retrospective review of our patients who underwent autologous fat grafting to the breast over a 2-year period (60). The volume of fat harvested, volume of fat injected after processing, time taken to complete fat grafting (from harvest to injection), and complications within 60 days of grafting were compared between the two processing methods. There were a total of 118 patients in the centrifugation and 103 patients in the REVOLVETM (LifeCell Corp.) group. We found that the mean volume of fat harvested and injected were significantly higher in the REVOLVETM (LifeCell Corp.) group and the time to complete fat grafting was significantly shorter, 30 vs. 85 min (Table 1). There were no complications in either group. These results suggest that the convenience of using a streamlined system for fat harvesting and processing allows for a larger volume of fat to be harvested and injected than would normally be the case using a conventional method. The all in one system also eliminates unnecessary fat handling time which could translate to reduced operative time.

Full table
Placement
The placement of the processed concentrated fat into a recipient site is one of the most challenging aspects of fat grafting. The general principle is to position small parcels of fat between layers of host tissue so as to encourage uniform survival, stability, and integration into the surrounding tissues (52). This could be particularly challenging in a postmastectomy reconstructed breast where host subcutaneous tissue has been voided. Thus, in this case, fat parcels are positioned between the overlying breast skin and the pectoralis major muscle. It is also generally understood that injecting a single bolus of a large volume of fat is to be avoided as this leads to fat necrosis and a poor outcome because of a lack of sufficient contact with vascularized host tissue.
Although there is no standardized fat placement technique, the Coleman technique is the most widely used (52). In this technique, fat is injected using a blunt Coleman infiltration cannula attached to a 1 mL syringe while withdrawing the cannula. Other syringe sizes (3 and 10 mL) as well as various cannula tip shapes, diameters, lengths, and curves may be used depending on the volume of fat to be placed and the recipient site. The use of wider-diameter cannulas (2.5 mm) may however be preferred as they have been shown to potentially improve fat graft survival and reduce fat graft resorption compared with small-diameter cannulas (1.6 or 2 mm) (41). Instead of cannulas, needles may also be used for fat injection. Evidence suggests that the size of the needles does not appear to affect cell viability, at least when using 14, 16, and 20 gauge needles (42). But for any given needle size, it appears that fat viability is influenced by the shear stress, which is a function of flow rate. Fat injected at a slow rate (low shear stress) results in better fat graft retention than fat injected at a fast rate (high shear stress) (57).
The volume of fat injected appears to be another important variable that may influence graft viability and retention. Choi et al. applied 3-dimensional imaging technology to assess volumetric fat graft survival following autologous fat transfer to the breast (61). They reported that patients receiving higher volumes (average of 151 cc) of injected fat had slower volume loss and greater total volume retention than those receiving smaller volumes (average of 51 cc). Moreover, the time from fat injection also impacted retention rates; the longer the time from fat injection, the lower the fat retention. In further evaluating the impact of graft volume, Del Vecchio et al. identified the graft-to-capacity ratio (defined as the volume of grafted fat in relation to the volume of the recipient site) as another important variable to consider (62). In 30 patients undergoing large-volume fat transplantation to the breast, the authors calculated the average graft-to-capacity ratio using pre-operative quantitative volumetric analysis (using 3-dimensional breast imaging), volume of fat injected, and post-operative quantitative volumetric breast imaging at 12 months. Patients whose graft-to-capacity ratio exceeded 1 standard deviation (SD) of the calculated average had a lower percentage of volume maintenance at 12 months. Conversely, those who had a graft-to-capacity ratio that was 1 SD lower than the average demonstrated a higher percentage of volume maintenance. Thus, an understanding of the biology and volumetric capacity of the recipient site may lead to more consistent outcomes following fat grafting.
In reconstructed breasts, prior irradiation is also an important variable that could impact graft retention. In an experimental study, human fat grafts injected into irradiated mice were shown to reduce radiation-induced fibrosis but fat graft retention was significantly lower than in nonirradiated tissue (63). In contrast to this study, two clinical studies have shown that prior irradiation to the breasts had no impact on fat retention (26,61).
In summary, current data suggests that fat should be ideally injected with low shear stress taking into consideration the volume or graft-to-capacity to optimize graft retention.
Authors’ tips
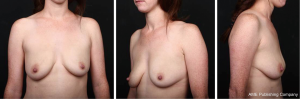
We have been using fat grafting for over 7 years to correct volume, shape, and contour deformities at the second stage of implant-based reconstructions in irradiated as well as nonirradiated breasts (Figure 2). As delineated in the review of the literature above, the outcome of fat grafting is highly dependent on the technique. Over the years we have refined our technique and established some principles:
- Handling of fat tissue. When handling fat tissue, surgeons need to be cognizant that fat tissue is living tissue. Delicate handling during harvesting, processing, and injection is of utmost importance to preserve its integrity. Exposure to inappropriate external forces, including mechanical, chemical, or barometric, should be avoided to minimize the risk of cellular damage and necrosis which could adversely affect graft viability and retention. In addition, the harvested fat should be maintained as close as possible to body temperature to maximize its survival;
- Preoperative planning. As with any surgical procedure, preoperative planning is important. A thorough patient evaluation should be performed that includes an assessment of the patient’s body habitus, prior breast surgeries, and any medical history that might complicate surgery. The amount of fat that would be required to address a particular breast deficit and the potential site of procurement should also be assessed and determined prior to the surgical procedure. In assessing for a suitable donor site, clinical judgment is needed to select a site that is likely to have a good outcome keeping in mind that sometimes donor site irregularities, as a secondary complication of fat grafting, may be more difficult to treat. In addition, one has to be mindful that a total autologous reconstruction may be needed depending on the patient’s cancer stage and breast reconstruction outcome, especially if the patient is considering it, and therefore the abdomen, at least the lower portion and peri-umbilical area should be preserved;
- Sterile technique. General principles of sterile technique should be observed at all stages of the procedure. Preoperative preparation using antimicrobial scrubs and prep solutions should be adhered to;
- Tumescent solution. In general, about 1 mL of tumescent solution is injected for every 1 mL of lipoaspirate to be extracted. At least 7 min is needed for the vasoconstrictive effects to set in before fat extraction can be performed. Our standard tumescent solution is 20 cc of 1% lidocaine and 1 ampule of epinephrine in 1 L of Lactated Ringer’s solution;
- Fat aspiration. We typically use a 3-4 mm cannula size, depending on the location of the donor site, with low-suction vacuum. Suction-assisted lipectomy is preferred as it allows for more control in setting the pressure and gentle movements are utilized to harvest the fat;
- Processed fat. At times, the processed fat is kept for a period of time prior to injection as the surgeon is busy performing another procedure. However, this can be detrimental to graft survival given that the fat has now lost the core body temperature that was harvested from. The goal is to harvest, process, and inject immediately;
- Fat injection. We advocate use of low-pressure when injecting the processed fat into the recipient site. However, increased pressure may sometimes be needed, for example, when injecting into scarred planes of tissues; but, it is important to be aware of the amount of pressure that is being exerted to inject the fat. High pressures on a plunger have a negative effect on fat survival;
- The efficacious combination of procurement, processing, and placement should always be considered during fat grafting.
Future of fat grafting
Given the versatility of fat tissue as well its biocompatibility, fat grafting will continue to be an important component of breast reconstruction. Although currently fat grafting is utilized almost exclusively as an adjunctive procedure in breast reconstruction (1), the stage is being set for its use as the primary means of reconstruction (2). However, fat grafting alone to reconstruct a breast cannot be feasible in all patients because of fat availability which is a major limitation. But, as surgeons become more comfortable with fat grafting and with technology evolving to simplify the process, we could see fat grating playing a more prominent role in breast reconstruction; for example, in partial substitution of the implant for fat (i.e., changing the ratio of implant volume to fat by using a smaller implant and substituting with a larger volume of fat). Again, fat availability would pose a limitation. Fat may also be combined with biologic scaffolds to create what we call “bioengineered breasts” (Figures 3,4). In this construct, an acellular matrix is used at the lower pole with a tissue expander in the first stage of reconstruction followed by submuscular placement of a second acellular matrix at the upper pole plus fat grafting along with an implant in the second stage. The combination of acellular matrix and autologous fat provides the soft tissue volume to address tissue deficiency. The acellular matrix placed submuscularly serves as a scaffold to support tissue regeneration, generating a layer of soft tissue at the upper pole while autologous fat grafting provides the extra padding to smooth out deficiencies in the breast shape/contour as well as mask deformities. This powerful combination of constructs will better allow us to achieve the ultimate goal of breast reconstruction―to recreate a breast that looks and feels like the natural breast.
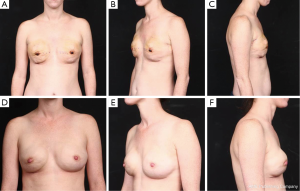
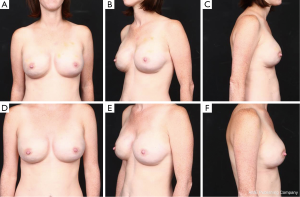
Conclusions
Although it is well acknowledged that the clinical outcome of fat grafting is dependent on the technique, a review of the published literature does not provide clear guidance as to the optimal technique at each of the stages of fat grafting. Nonetheless, the use of lower abdomen and medial thigh as donor sites, use of low suction pressure for liposuction, use of large bore-sized harvesting cannulas, use of low centrifugation forces (if using centrifugation for processing), use of low shear stress during injection, placement of small parcels of fat, and optimizing the volume of fat injected to the capacity of the recipient site were noted to be associated with improved fat retention. Surgeons should be cognizant that the injected fat tissue has to survive at times in a hostile recipient site. Thus, every effort needs to be made to enhance graft take and all the factors mentioned above should be taken into consideration. In addition, one must not forget that maintaining the fat as close to core body temperature as possible and immediate grating following harvest also enhances graft take. Clearly, rigorous, controlled studies are needed to determine optimal grafting conditions; but, for now surgeons may have to rely on optimizing their technique of choice.
Acknowledgements
We would like to thank Kalanethee Paul-Pletzer, PhD (LifeCell Corporation, Branchburg, NJ) for providing Medical Writing support for this manuscript.
Disclosure: Allen Gabriel, MD and G. Patrick Maxwell, MD are consultants for LifeCell Corporation, Branchburg, NJ.
References
- Kling RE, Mehrara BJ, Pusic AL, et al. Trends in autologous fat grafting to the breast: a national survey of the american society of plastic surgeons. Plast Reconstr Surg 2013;132:35-46. [PubMed]
- Khouri RK, Rigotti G, Khouri RK Jr, et al. Total breast reconstruction with autologous fat transfer: review of a seven-year multicenter experience. Plast Reconstr Surg 2014;134:84-5. [PubMed]
- Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg 2005;116:1300-5. [PubMed]
- Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthetic Plast Surg 2009;33:706-15. [PubMed]
- Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: the management of acquired contour deformities. Plast Reconstr Surg 2009;124:409-18. [PubMed]
- de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg 2011;128:411e-8e. [PubMed]
- Losken A, Pinell XA, Sikoro K, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg 2011;66:518-22. [PubMed]
- Del Vecchio DA. "SIEF"--simultaneous implant exchange with fat: a new option in revision breast implant surgery. Plast Reconstr Surg 2012;130:1187-96. [PubMed]
- Cigna E, Ribuffo D, Sorvillo V, et al. Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci 2012;16:1729-34. [PubMed]
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409-22; discussion 1423-4. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg 2012;129:317-29. [PubMed]
- Sarfati I, Ihrai T, Kaufman G, et al. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J Plast Reconstr Aesthet Surg 2011;64:1161-6. [PubMed]
- Caviggioli F, Maione L, Forcellini D, et al. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg 2011;128:349-52. [PubMed]
- Delay E, Streit L, Toussoun G, et al. Lipomodelling: an important advance in breast surgery. Acta Chir Plast 2013;55:34-43. [PubMed]
- Niechajev I, Sevćuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 1994;94:496-506. [PubMed]
- Zocchi ML, Zuliani F. Bicompartmental breast lipostructuring. Aesthetic Plast Surg 2008;32:313-28. [PubMed]
- Gutowski KA; ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 2009;124:272-80. [PubMed]
- Padoin AV, Braga-Silva J, Martins P, et al. Sources of processed lipoaspirate cells: influence of donor site on cell concentration. Plast Reconstr Surg 2008;122:614-8. [PubMed]
- Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 2013;382:1113-20. [PubMed]
- Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg 2010;64:222-8. [PubMed]
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 2008;34:1178-85. [PubMed]
- Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 2006;12:3375-82. [PubMed]
- Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg 2004;113:391-5; discussion 396-7. [PubMed]
- Ullmann Y, Shoshani O, Fodor A, et al. Searching for the favorable donor site for fat injection: in vivo study using the nude mice model. Dermatol Surg 2005;31:1304-7. [PubMed]
- Li K, Gao J, Zhang Z, et al. Selection of donor site for fat grafting and cell isolation. Aesthetic Plast Surg 2013;37:153-8. [PubMed]
- Small K, Choi M, Petruolo O, et al. Is there an ideal donor site of fat for secondary breast reconstruction? Aesthet Surg J 2014;34:545-50. [PubMed]
- Geissler PJ, Davis K, Roostaeian J, et al. Improving fat transfer viability: the role of aging, body mass index, and harvest site. Plast Reconstr Surg 2014;134:227-32. [PubMed]
- Moore JH Jr, Kolaczynski JW, Morales LM, et al. Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg 1995;19:335-9. [PubMed]
- Kim IH, Yang JD, Lee DG, et al. Evaluation of centrifugation technique and effect of epinephrine on fat cell viability in autologous fat injection. Aesthet Surg J 2009;29:35-9. [PubMed]
- Shoshani O, Berger J, Fodor L, et al. The effect of lidocaine and adrenaline on the viability of injected adipose tissue--an experimental study in nude mice. J Drugs Dermatol 2005;4:311-6. [PubMed]
- Keck M, Janke J, Ueberreiter K. Viability of preadipocytes in vitro: the influence of local anesthetics and pH. Dermatol Surg 2009;35:1251-7. [PubMed]
- Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg 2010;126:1500-5. [PubMed]
- Leong DT, Hutmacher DW, Chew FT, et al. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci 2005;37:169-76. [PubMed]
- Pu LL, Coleman SR, Cui X, et al. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg 2008;122:932-7. [PubMed]
- Ferguson RE, Cui X, Fink BF, et al. The viability of autologous fat grafts harvested with the LipiVage system: a comparative study. Ann Plast Surg 2008;60:594-7. [PubMed]
- Crawford JL, Hubbard BA, Colbert SH, et al. Fine tuning lipoaspirate viability for fat grafting. Plast Reconstr Surg 2010;126:1342-8. [PubMed]
- Gonzalez AM, Lobocki C, Kelly CP, et al. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg 2007;120:285-94. [PubMed]
- Rohrich RJ, Morales DE, Krueger JE, et al. Comparative lipoplasty analysis of in vivo-treated adipose tissue. Plast Reconstr Surg 2000;105:2152-8; discussion 2159-60. [PubMed]
- Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 2001;27:819-26. [PubMed]
- Fisher C, Grahovac TL, Schafer ME, et al. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg 2013;132:351-61. [PubMed]
- Ozsoy Z, Kul Z, Bilir A. The role of cannula diameter in improved adipocyte viability: a quantitative analysis. Aesthet Surg J 2006;26:287-9. [PubMed]
- Erdim M, Tezel E, Numanoglu A, et al. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg 2009;62:1210-4. [PubMed]
- Cheriyan T, Kao HK, Qiao X, et al. Low harvest pressure enhances autologous fat graft viability. Plast Reconstr Surg 2014;133:1365-8. [PubMed]
- Allen RJ Jr, Canizares O Jr, Scharf C, et al. Grading lipoaspirate: is there an optimal density for fat grafting? Plast Reconstr Surg 2013;131:38-45. [PubMed]
- Salinas HM, Broelsch GF, Fernandes JR, et al. Comparative analysis of processing methods in fat grafting. Plast Reconstr Surg 2014;134:675-83. [PubMed]
- Butterwick KJ. Lipoaugmentation for aging hands: a comparison of the longevity and aesthetic results of centrifuged versus noncentrifuged fat. Dermatol Surg 2002;28:987-91. [PubMed]
- Khater R, Atanassova P, Anastassov Y, et al. Clinical and experimental study of autologous fat grafting after processing by centrifugation and serum lavage. Aesthetic Plast Surg 2009;33:37-43. [PubMed]
- Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg 2010;63:1375-81. [PubMed]
- Botti G, Pascali M, Botti C, et al. A clinical trial in facial fat grafting: filtered and washed versus centrifuged fat. Plast Reconstr Surg 2011;127:2464-73. [PubMed]
- Ramon Y, Shoshani O, Peled IJ, et al. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast Reconstr Surg 2005;115:197-201; discussion 202-3. [PubMed]
- Minn KW, Min KH, Chang H, et al. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg 2010;34:626-31. [PubMed]
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg 2001;28:111-9. [PubMed]
- Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 2008;121:1033-41; discussion 1042-3. [PubMed]
- Hoareau L, Bencharif K, Girard AC, et al. Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiency. J Plast Reconstr Aesthet Surg 2013;66:712-9. [PubMed]
- Xie Y, Zheng D, Li Q, et al. The effect of centrifugation on viability of fat grafts: an evaluation with the glucose transport test. J Plast Reconstr Aesthet Surg 2010;63:482-7. [PubMed]
- Pulsfort AK, Wolter TP, Pallua N. The effect of centrifugal forces on viability of adipocytes in centrifuged lipoaspirates. Ann Plast Surg 2011;66:292-5. [PubMed]
- Lee JH, Kirkham JC, McCormack MC, et al. The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg 2013;131:1125-36. [PubMed]
- Zhu M, Cohen SR, Hicok KC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg 2013;131:873-80. [PubMed]
- Ansorge H, Garza JR, McCormack MC, et al. Autologous fat processing via the Revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J 2014;34:438-47. [PubMed]
- Maxwell G, Gabriel A. REVOLVE autologous fat processing system reduces operative time. California Society of Plastic Surgeons 64th Annual Meeting. Newport Beach, CA, May 2014.
- Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg 2013;131:185-91. [PubMed]
- Del Vecchio DA, Del Vecchio SJ. The graft-to-capacity ratio: volumetric planning in large-volume fat transplantation. Plast Reconstr Surg 2014;133:561-9. [PubMed]
- Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg 2014;134:249-57. [PubMed]