Current perspectives on radiation therapy in autologous and prosthetic breast reconstruction
Introduction
Recent trends in the treatment of breast cancer include increased mastectomies both bilateral and prophylactic procedures, conservation of skin and nipple tissue, and expanded indications for the use of radiation therapy (1-4). Radiation therapy is an important adjunct in the treatment of breast cancer by eliminating subclinical disease in combination with surgical removal of gross tumor (5). This has led to a number of evolving therapeutic implications and operative considerations for reconstructive surgeons.
In general, autologous tissue tends to be superior to implant-based reconstructions in the setting of postmastectomy radiation therapy (PMRT) (6). Autologous reconstructions that can be delayed until after PMRT avoid radiation-induced sequelae; however this approach is not always feasible. Prosthetic reconstruction of the irradiated breast is more challenging, results in lower patient satisfaction, and is heavily dependent upon timing of staged procedures. However, improved aesthetic outcomes are increasingly possible with the development of breast implant innovations, acellular dermal matrices (ADM), and fat grafting (7,8). In 2012, the senior author published two reviews in Plastic and Reconstructive Surgery on radiation therapy and prosthetic and autologous breast reconstruction (9,10). The purpose of this article is to update the previous literature reviews and revise recommendations for breast reconstruction in the setting of PMRT.
Overview of literature evaluation
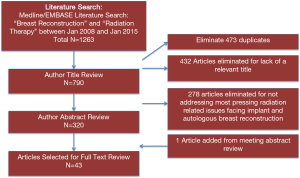
A search and review of the MEDLINE and EMBASE databases was performed for articles published between January of 2008 and January of 2015 on breast reconstruction and radiation therapy (Figure 1). Relevant studies were assigned a level of evidence using the American Society of Plastic Surgeons (ASPS) Evidence Rating Scale for Therapy (11). Using the search terms, “radiation therapy” and “breast reconstruction” the query revealed 1,263 articles. A total of 473 articles were removed as duplicates and 432 were removed for lack of relevance. A title review was performed on the remaining 790 articles, and 278 were eliminated due to not directly addressing the search criteria. An abstract review was performed of the 320 remaining articles. Forty-three articles were selected for full text review, and bibliography review yielded an additional article from a meeting abstract (Table 1). This subgroup of articles was reviewed in detail. A total of 16 papers provided level III evidence; 10 manuscripts provided level I or II evidence. Seventeen case series provided level IV evidence and were included because they presented novel perspectives. The majority of studies focused on the injurious effects of radiation therapy and increased complications and concomitant lower patient satisfaction with reconstruction.
Full table
Impact of radiation on the reconstructed breast
Radiation increases the risk of complications, need for reoperations, and worsens aesthetic outcomes in breast reconstruction. In a retrospective review of 1,037 breast reconstruction patients, Barry and colleagues reported that tissue expander reconstructions had a major complication rate of 24.4% without radiation therapy and 45.4% with radiation therapy (23). The authors noted that only 70.1% implant-based reconstructions in the setting of PMRT were able to retain the implant and that 10.3% of the explantations would ultimately require an autologous reconstruction. Radiation was the greatest risk factor for major complications in tissue expander/implant reconstruction (level III evidence). However, among autologous reconstructions, multivariate analysis revealed no statistically significant difference in rates of major complications between patients receiving preoperative radiation therapy and those who did not (P=0.84). Another study utilizing the BREAST-Q reconstruction questionnaire investigated patient satisfaction in 482 patients undergoing implant breast reconstruction (24). A multivariate model demonstrated that prior radiation therapy (P<0.001) or PMRT (P=0.002) had a significantly negative effect on patient satisfaction (level I evidence). Sbitany and colleagues reviewed 903 immediate two stage breast reconstructions and found that any radiation delivery caused an increased rate of infection requiring antibiotics (21.6%, P<0.001) and an increased risk of expander/implant loss (18.75%, P=0.03) (14). Prior history of radiation had a higher risk of wound breakdown (P=0.012) (level III evidence). The authors concluded that both preoperative radiation and PMRT in immediate implant-based reconstruction resulted in higher, albeit acceptable, complication risks.
Ho and colleagues reported their experience in immediate 2-stage tissue expander to implant reconstruction 604 patients, with 113 receiving PMRT (19). They noted a 4.2 increased odds (P=0.001) of major complications in the irradiated group. Grade III and IV capsular contracture rate was significantly higher in the irradiated group compared with matched controls (21.7% vs. 10%; P<0.008) (level III evidence). PMRT to tissue expanders is associated with high complications.
Radiation therapy and nipple sparing mastectomy
Spear and colleagues evaluated prosthetic reconstruction of nipple sparing mastectomies in the setting of radiation therapy. Of 18 patients, 72.2% had previous breast conserving therapy (BCT) with RT and 27.8% underwent PMRT. With an average follow-up of 3 years, patients were reported to have 33.3% first-stage complications and most common indications for revision were for correction of implant malposition (27.8%), capsular contracture (16.7%), and nipple malposition (22.4%). Capsular contracture occurred more commonly in patients who needed PMRT compared with those who had previously undergone breast-conservation therapy (40% vs. 7.8%). The authors found that a reconstruction was maintained or at least salvage in 88.9% patients, and only 11.1% of patients completely lost their implant.
The combination of nipple-sparing mastectomy, implant reconstruction, and radiation therapy results in an obviously high complication rate and high likelihood of revisionary surgery. While the authors concluded that nipple/areola complex preservation is safe in women undergoing radiation therapy, prosthetic complication rates of these challenging patients is similar to non-radiated patients. Further studies are warranted to determine if autologous reconstruction is superior and/or confers any protective benefit to the reconstruction in comparison to prosthetic reconstruction (level IV evidence).
Burdge and colleagues found similar results in their review of 1,035 mastectomies (558 NSSM and 477 SSM) (17). For prosthetic reconstruction, NSSM had higher rates of wound infection, tissue necrosis, and expander when compared to patients with SSM. For both direct to implant immediate reconstructions or two stage tissue expander to implant reconstructions, overall radiation induced complication rate was 38.1% in NSSM and 30.8% for SSM. The authors found that oncologic outcomes were similar for NSSM and SSM, and that prosthetic reconstruction can be performed in NSSM in the setting of radiation but has a higher complication risk.
Radiation therapy and delivered before reconstruction
While the effects of radiation are well-established, debate exists over whether previous radiation therapy in BCT may not be as detrimental to a reconstruction as PMRT. Patients treated with previous radiation therapy in BCT do not have the same complication profiles as patients receiving PMRT. Hirsch et al. reported on a series of 876 tissue expander to implant breast reconstructions to determine complication profiles by stage of reconstruction (12). The authors found that during final implant placement, any history of radiation had the strongest association with the development of complications leading to explantation and/or conversion to an autologous flap (OR =3.45). Risk factors associated with complications in either stage 1 or 2 were age greater than 50 years, active smoking, and a history of BCT with RT or PMRT (level III evidence).
Similarly, a study of 717 patients from the Danish Registry for Plastic Surgery evaluated the effect of radiation therapy timing on capsular contracture and reoperations in 1- or 2-stage prosthetic breast reconstruction (25). Radiation therapy was significantly associated with capsular contracture after both 1-stage [adjusted hazard ratio (HR) =3.3; 95% CI, 0.9-12.4] and 2-stage procedures (HR =7.2; 95% CI, 2.4-21.4), and risk of reoperation after both 1-stage (HR =1.4; 95% CI, 0.7-2.5) and 2-stage procedures (HR =1.6; 95% CI, 0.9-3.1). In the setting of radiation for 2 stage procedures, reconstruction failure was 13.2% (level II evidence). The data strongly suggests employing alternatives to prosthetic reconstruction in the setting of radiation therapy.
Hypofractionation is the delivery of radiation therapy in fewer albeit larger daily fractions. Hypofractonation was developed in an effort to improve local regional recurrence while maintaining acceptable cosmetic results and patient morbidity. Whitfield and colleagues reported on the reconstructive outcomes of using a 40 Gy in 15 fractions over 3 weeks protocol of radiation delivery (26). A total of 178 patients underwent implant-based breast reconstruction. The actuarial rates of severe capture contracture at 1, 2, 3, 4, 5, and 6 years of follow-up were 0%, 5%, 5%, 21%, 30%, and 30% whereas the nonirradiated group had no cases of severe capsular contracture (P<0.001) (level III evidence). Khansa et al. investigated the effect of prior BCT on patient satisfaction in 802 breast reconstructions (27). Previous BCT with RT had higher rates of skin flap necrosis (12.4% vs. 6.8%, P=0.024) but did not higher rates of other complications or lower rates of satisfaction with aesthetic outcomes (Level III evidence). Definitive conclusions are difficult draw given this cohort only had ten patients. A severe limitation of all studies evaluating the effect of previous BCT and RT is to treat these patients as a homogenous cohort. There is likely a reparative process that occurs so that after a sufficient amount of years, complication risk in BCT patients might very well fall to levels consistent with radiation-naïve patients, however without sufficient data addressing timing of BCT to subsequent mastectomy, this remains speculative.
Radiation therapy delivered after reconstruction
The effect of PMRT has a more significant impact on complications and failure of reconstruction than previous radiation with BCT. A study of the Danish Plastic Surgery Registry evaluated outcomes of direct to implant reconstruction at the time of mastectomy and found that patients who received PMRT had significantly increased revisions (P=0.047) and lower aesthetic scores (level IV evidence) (28).
A prospective, multi-institutional study evaluated factors associated with reconstruction failure in 141 consecutive patients undergoing mastectomy and immediate 2-stage breast reconstruction and PMRT (29). After a median follow-up time of 37 months, 67.5% of patients had Baker I or II capsular contracture and 32.5% of patients had a Baker III or IV. Multiple regression analysis revealed T3 or T4 tumor, smoking, and positive axillary nodes were associated with reconstructive failure (level II evidence).
Jhaveri and colleagues reported long-term outcomes and aesthetic results in either two-stage prosthetic (69 patients) or autologous reconstructions (23 patients) (30). Major complication rate was 33.3% for prosthetic reconstruction vs. 0% for autologous reconstruction (P=0.001). The rate of minor complications was 55% for prosthetic reconstruction vs. 8.7% for autologous reconstruction (P<0.001). Acceptable cosmesis was only 51% of prosthetic patients compared to 82.6% of autologous patients (P=0.007) (level II evidence). These results demonstrate that implant-based reconstruction is associated with more major and minor long-term complications and worse cosmetic results than autologous reconstruction.
McKeown and colleagues reported on the effect of timing of radiation therapy on reconstruction with LD flaps and implants (31). A total of 13 patients who underwent immediate reconstruction followed by radiation therapy and were compared to 11 patients who underwent radiation therapy followed by delayed reconstruction. The authors noted a trend towards better long-term cosmetic outcome in patients undergoing delayed reconstruction, with volume and contour of the upper pole being most negatively affected by radiation (level II evidence).
Barry et al. performed a meta-analysis evaluating optimal sequencing of breast reconstruction and PMRT (32). A review of 1,105 patients from 11 studies demonstrated that the rate of adverse events was 4.2 times as high in patients undergoing PMRT as it is in patients not undergoing PMRT. When PMRT was delivered after immediate breast reconstruction, patients who had autologous tissue-based reconstruction had one-fifth the risk of adverse events of patients who had implant-based reconstruction. A similar pattern was seen when PMRT was delivered before delayed breast reconstruction (level III evidence). The results suggest that autologous reconstructions have superior outcomes to prosthetic reconstructions whether performed immediately or in a delayed fashion.
Evaluating post-radiation skin changes to predict complication rates
Parsa and colleagues hypothesized that an objective evaluation of post-radiation skin changes based upon a novel classification system could help guide surgeons as to which patients may be suitable candidates for a prosthetic reconstruction (33). In patients whose chest walls displayed moderate skin changes without induration after irradiation, aesthetic outcomes after reconstruction were similar on the irradiated and nonirradiated sides (P>0.50). In contrast, in patients who developed induration or severe post-radiation skin changes, the rate of modified Baker IV capsular contracture was higher on the irradiated side, and the rate of poor aesthetic outcomes on the irradiated side was 75% in patients with severe skin changes and 100% in those with induration (level II evidence). While most reports suggest autologous reconstructions are preferable in the setting of radiation therapy, patients may be stratified as an acceptable prosthetic candidate based upon skin response to radiation therapy.
Consequence of a tissue expander, implant, or autologous flap on radiation delivery and oncologic outcomes
There is ongoing concern over the oncologic safety of radiation therapy and tissue expanders on the chest wall and whether a metallic port interferes with delivery particularly in patients where the internal mammary nodes require treatment. Kronowitz and colleagues reported that the presence of a tissue expander on the chest wall during radiation therapy does not impact recurrence-free survival (34). Locoregional recurrence risk was compared between 47 patients with advanced breast cancer with a tissue expander receiving PMRT and 47 disease-matched control patients who were treated with PMRT and no tissue expander. The 3-year recurrence-free survival rates were equal and there was no locoregional recurrence in the tissue expander cohort at a median follow-up time of 40 months. The 3-year recurrence-free survival rates were 92% for the tissue expander cohort compared to 86% for the control group (P=0.87) (level II evidence). Several important conclusions were emphasized by the authors. Full-height expanders should be avoided as they may theoretically interfere with radiation treatment of the clavicular nodal basins. If an expander requires deflation for PMRT, only partially deflate between one third and one half the expander volumes. Reinflation should be performed within 2 weeks post-radiation to preserve the skin envelope. For patients in whom the internal mammary nodes need to be treated with an implant on the chest wall, higher doses of radiation may have to be delivered to the heart and lungs, which may theoretically increase the risk of coronary artery disease and pulmonary fibrosis.
Radiated flaps clearly have worse aesthetic outcomes but some authors argued that an autologous flap interfere with radiation fields, especially the internal mammary lymph nodes (35,36). While routine treatment of internal mammary lymph nodes has not gained widespread support among radiation oncologists, the National Cancer Institute of Canada Clinical Trials Groups MA-20 study reported on a prospective randomized trial of patients with one to three positive lymph nodes treated with whole breast irradiation with or without regional nodal irradiation after segmental mastectomy (37). The study demonstrated that regional nodal irradiation resulted in a 30% relative improvement in disease-free survival, 41% lower rate of regional recurrence, and a 36% lower rate of distant recurrence. These findings are increasingly applied to mastectomy patients to receive routine delivery of PMRT to the regional nodal basins, including the internal mammary chain. National trends among medical centers are still evolving and will have important implications for reconstructive surgeons.
Crisera and colleagues addressed the oncologic safety of performing immediate free flap reconstruction for advanced-stage breast cancer (stage IIB or greater) (20). The authors performed a retrospective cohort study of 170 patients with skin sparing mastectomy with immediate free flap breast reconstruction, and found that PMRT did not adversely affect local disease recurrence or overall survival rates. Radiation therapy was administered to 131 patients (28 preoperatively and 103 postoperatively) and local recurrences were noted in 15 patients (8.8%) after a median of 22.9 months (range, 3.0-59.2 months). A total of 13 patients experienced moderate to severe flap distortion/shrinkage, and an additional salvage flap was required in seven patients to correct deformities. It is important to note that the administration of postoperative chemotherapy was delayed in eight patients (4.7%) because of wound healing complications (level III evidence). Although performing immediate breast reconstruction with autologous tissue before PMRT has been shown to be oncologically safe, doing so subjects patients to higher rates of fat necrosis and diminished aesthetic outcomes.
Need for corrective surgery in irradiated reconstructed breasts
Ho and colleagues focused on the rates of permanent implant removal or replacement (PIRR) surgery following radiation therapy in a retrospective review of 751 patients receiving an immediate tissue expander placement (18). Of these, 151 patients went on to receive chemotherapy and exchange to a permanent implant, followed by PMRT. The 7-year PIRR free rate was 71%. The 7-year rate of implant replacement was 17.1% and removal was 13.3%. Most frequent reasons for implant removal included infection, implant extrusion, and malposition. Of note, two patients experienced local recurrence in the chest wall, both after 7 years and the 7-year distant metastasis-free survival rate was 81% and overall survival 93% (level III evidence). Prosthetic reconstruction followed by PMRT is associated with high rates of long-term device replacement and revision.
At the same institution, Cordeiro and colleagues reported a single surgeon experience of 2,133 prosthetic breast reconstructions with 319 receiving PMRT (16). Implant loss occurred in 9.1% of irradiated implants compared to just 0.5% of nonirradiated implants (P<0.01). Capsular contracture grade IV was present in 6.9% of irradiated compared to just 0.5% of nonirradiated implants (P<0.01). Predicted implant loss rates were 17.5% and 2.0% for irradiated and nonirradiated implants, respectively, at 12 years (P<0.01) (level III evidence).
Traditionally, surgeons have delayed final reconstruction until after the administration of radiation therapy to avoid the damaging effects of ionizing radiation on the reconstruction. Garvey and colleagues evaluated whether certain types of autologous reconstructions could better withstand the effects of radiation therapy over others for development of fat necrosis and need for revision (15). The 625 flaps were analyzed, 6.4% irradiated vs. 93.6% non-irradiated. Overall complication rates were similar for both the irradiated and nonirradiated flaps. Irradiated flaps [i.e., both DIEP and muscle-sparing free transverse rectus abdominis myocutaneous (TRAM)] flaps developed fat necrosis at a significantly higher rate (22.5%) than the nonirradiated flaps (9.2%, P=0.009). There were no differences in fat necrosis rates between the DIEP and muscle-sparing free TRAM flaps in both the irradiated and nonirradiated groups. Surprisingly, there was no statistically significant difference in the need for reoperative surgery for fat necrosis between the irradiated and nonirradiated flaps (level III evidence).
Classen et al. assessed fibrosis and capsular contracture of breast reconstructions subjected to radiation therapy in 109 patients (38). The median radiation therapy dose was 50.4 Gy and 44 patients received a boost dose of 10 Gy. Eighty-two patients had implant-based reconstructions, 20 had autologous tissue-based reconstructions, and 7 had combined reconstructions. After a mean follow-up time of 34 months, the 3-year incidence of ≥ grade III fibrosis was 20 percent for the implant-based reconstructions and 43% overall. The 3-year rate of surgical correction of the contralateral breast was 30%, and 39 patients (35.8%) required unplanned surgery on the reconstructed breast (level IV evidence).
Wong and colleagues evaluated revision surgery in 62 patients undergoing mastectomy, immediate reconstruction, followed by radiation therapy (39). Major corrective surgery was 40% (6/15 reconstructions) in the implant group and 9% (4/47) in the nonimplant group (P=0.01) (level III evidence). Patients who undergo mastectomy and immediate implant-based reconstruction followed by PMRT are at high risk for needing subsequent major corrective surgery.
Autologous fat grafting to salvage radiated reconstructions
Panettiere et al. evaluated whether fat grafting could salvage prosthetic reconstructions after irradiation (40). The study included 61 patients with twenty requiring multiple sessions of lipofilling, compared to 41 controls with no fat grafting. Fat grafted patients were significantly better aesthetic scores than those before fat grafting and were also significantly better than those for the untreated control breasts (level II evidence).
Serra-Renom and colleagues reported on outcomes of injecting autologous fat grafts during delayed expander placement after PMRT (41). In 65 patients, a tissue expander was inserted endoscopically under the pectoralis major muscle and underwent total immediate expansion. Next, a mean of 150±25 cc of autologous fat was injected to superior quadrants between the skin and the pectoralis muscle. Exchange to a permanent implant was performed at three months with an additional injection of a mean of 150±30 cc of fat to the lower quadrants. At 1 year follow-up, patients’ mean satisfaction rating was 4 (Scale: 1—low to 5—high); and there were no cases of capsular contracture greater than Baker I (level II evidence). Fat grafting may have a role in thickening mastectomy skin flaps over an implant which may aid in improving radiation sequelae and fibrosis.
Losken and colleagues evaluated the need for autologous fat grafting to TRAM flaps versus irradiated TRAM breast reconstructions (42). While contour, shape, and increase volume could be achieved in either cohort group, irradiated TRAM flaps required a significantly increased incidence of repeated injections (36% vs. 18%, P=0.002) (level IV evidence). While fat grafting may be beneficial in salvaging an irradiated flap, patients frequently require multiple sessions to achieve similar non-radiated results.
Use of acellular dermal matrix (ADM) in the setting of radiation therapy
Over the past decade, ADM have gained in popularity for purported benefits of improved pocket control, faster expansion, lowered capsular contracture rates, and improved aesthetic results albeit at a significant monetary cost. We performed a metanalysis to evaluate the clinical impact of radiation therapy on ADM-assisted breast reconstruction (13). In a review of 276 irradiated patients, ADMs in implant-based breast reconstruction in the setting of radiation therapy did not predispose to higher infection or overall complication rates or prevent bioprosthetic mesh incorporation. However, the rate of mesh incorporation may be slowed (level III evidence). Use of ADM for implant-based breast reconstruction does not appear to increase or decrease the risk of complications beyond nonirradiated ADM patients, but it may provide aesthetic benefits in properly selected patients.
Myckatyn and colleagues corroborated that the ADM remodeling process may be adversely impacted in patients who require radiation therapy, which can influence neovascularization and cellular proliferation (21). In biopsy specimens collected from 86 women undergoing exchange of a tissue expander for a breast implant, the authors found that chemotherapy with or without radiation adversely impacted type I collagen (P=0.02), cellular infiltration (P<0.01), extracellular matrix deposition (P<0.04), and neovascularization (P<0.01). Radiation exacerbated the adverse impact of chemotherapy. Neoadjuvant chemotherapy also caused a reduction in type I (P=0.01) and III collagen (P=0.05), extracellular matrix deposition (P=0.03), and scaffold degradation (P=0.02) (level III evidence).
Craig and colleagues reported a retrospective review of 1,376 immediate tissue expander breast reconstructions in four cohorts: ADM without and without radiation therapy, and non-ADM with and without radiation therapy (22). Overall complication rate between ADM and non-ADM cohorts were 39% and 16.7% respectively (P<0.001). Incidence of seroma tended to be higher in the ADM cohort and highest within patients that did receive RT when compared to non-ADM (13.6% vs. 10.9%, P>0.001). However, incidence of explantation was highest in the non-ADM with radiation therapy when compared to the ADM with radiation therapy group (20.4% vs. 11.4%, P=0.0012) (level III evidence). While overall complication rates, infection, and seroma tend to be higher with the use of ADMs, if recognized and appropriately treated, the expander reconstruction is often salvaged. ADM’s, once incorporated, may play a protective role in preventing the need for re-operations and explantations in patients undergoing radiation therapy.
Summary and clinical impact of the evidence
PMRT has a significant adverse impact on both short term and long term complication rates, aesthetic outcomes, and patient satisfaction with breast reconstruction. Most studies find a significant need for unplanned or major corrective surgery in irradiated breasts reconstructed with implants. However, with proper patient selection, acceptable complication rates are possible and the majority of patients who undergo implant-based reconstruction and PMRT ultimately keep their implant-based reconstruction with only a minority of patients requires conversion to an autologous tissue flap.
In the setting of PMRT, implant-based reconstructions are associated with a higher incidence of major corrective surgery than autologous tissue-based reconstruction. However, superior aesthetic outcomes are achieved with delayed reconstruction after PMRT than with immediate reconstruction before PMRT because of lower rates of fat necrosis, as well as improved volume and contour in the upper pole of the reconstructed breast.
The presence of a tissue expander, permanent implant, or autologous flap on the chest wall did not impede radiation delivery or have a significant effect breast cancer recurrence. Autologous fat grafting and ADMs have gained in popularity and may play a protective or restorative role in radiated breast reconstruction, capsular contracture, and aesthetic outcomes.
In conclusion, advances in plastic surgical technique have helped to mitigate trends in the expansion of radiation therapy. With modern implants and focused radiotherapy regimens, expander and implant related complications can be diminished to acceptable ranges in select patients. However, autologous reconstruction performed in a delayed fashion after PMRT remains a workhorse in these challenging patients. Despite these hurdles, it is critical that patients are not dissuaded from receiving reconstructive surgery and denied its important quality of life benefit simply because of their need for radiation therapy.
Acknowledgements
Dr. Clemens has consulted for Allergan Medical (Irvine, CA). Dr. Kronowitz has participated on a Scientific Advisory Committee for Allergan Medical.
Funding: Data analyses were supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Disclosure: The authors declare no conflict of interest.
References
- Keating NL, Landrum MB, Brooks JM, et al. Outcomes following local therapy for early-stage breast cancer in non-trial populations. Breast Cancer Res Treat 2011;125:803-13. [PubMed]
- Beahm EK, Walton RL. Issues, Considerations, and Trends in Bilateral Breast Reconstruction. Plast Reconstr Surg 2009;1064-76.
- Spear SL, Shuck J, Hannan L, et al. Evaluating long-term outcomes following nipple-sparing mastectomy and reconstruction in the irradiated breast. Plast Reconstr Surg 2014;133:605e-14e. [PubMed]
- Shirvani SM, Pan IW, Buchholz TA, et al. Impact of evidence-based clinical guidelines on the adoption of postmastectomy radiation in older women. Cancer 2011;117:4595-605. [PubMed]
- Yang TJ, Ho AY. Radiation therapy in the management of breast cancer. Surg Clin North Am 2013;93:455-71. [PubMed]
- Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg 2009;124:395-408. [PubMed]
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 2012;68:446-50. [PubMed]
- Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 2012;130:1-9. [PubMed]
- Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2012;130:513e-23e. [PubMed]
- Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2012;130:282-92. [PubMed]
- Haase SC, Chung KC. An evidence-based approach to treating thumb carpometacarpal joint arthritis. Plast Reconstr Surg 2011;127:918-25. [PubMed]
- Hirsch EM, Seth AK, Kim JY, et al. Analysis of risk factors for complications in expander/implant breast reconstruction by stage of reconstruction. Plast Reconstr Surg 2014;134:692e-9e. [PubMed]
- Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg 2012;130:27S-34S. [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg 2014;134:396-404. [PubMed]
- Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from postmastectomy radiation damage compared with the DIEP flap. Plast Reconstr Surg 2014;133:223-33. [PubMed]
- Cordeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg 2014;134:588-95. [PubMed]
- Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 2013;20:3294-302. [PubMed]
- Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 2012;118:2552-9. [PubMed]
- Ho AL, Bovill ES, Macadam SA, et al. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg 2014;134:1e-10e. [PubMed]
- Crisera CA, Chang EI, Da Lio AL, et al. Immediate free flap reconstruction for advanced-stage breast cancer: is it safe? Plast Reconstr Surg 2011;128:32-41. [PubMed]
- Myckatyn TM, Cavallo JA, Sharma K, et al. The impact of chemotherapy and radiation therapy on the remodeling of acellular dermal matrices in staged, prosthetic breast reconstruction. Plast Reconstr Surg 2015;135:43e-57e. [PubMed]
- Craig ES, Clemens MW, Koshy J, et al. Outcomes of acellular dermal matrix for immediate tissue expander reconstruction with radiotherapy. Plast Reconstr Surg 2014;134:83-4. [PubMed]
- Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol 2010;17 Suppl 3:202-10. [PubMed]
- McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer 2010;116:5584-91. [PubMed]
- Hvilsom GB, Hölmich LR, Steding-Jessen M, et al. Delayed breast implant reconstruction: is radiation therapy associated with capsular contracture or reoperations? Ann Plast Surg 2012;68:246-52. [PubMed]
- Whitfield GA, Horan G, Irwin MS, et al. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol 2009;90:141-7. [PubMed]
- Khansa I, Colakoglu S, Curtis MS, et al. Postmastectomy breast reconstruction after previous lumpectomy and radiation therapy: analysis of complications and satisfaction. Ann Plast Surg 2011;66:444-51. [PubMed]
- Roostaeian J, Pavone L, Da Lio A, et al. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg 2011;127:1407-16. [PubMed]
- Gross E, Hannoun-Levi JM, Rouanet P, et al. Evaluation of immediate breast reconstruction and radiotherapy: factors associated with complications. Cancer Radiother 2010;14:704-10. [PubMed]
- Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys 2008;72:859-65. [PubMed]
- McKeown DJ, Hogg FJ, Brown IM, et al. The timing of autologous latissimus dorsi breast reconstruction and effect of radiotherapy on outcome. J Plast Reconstr Aesthet Surg 2009;62:488-93. [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [PubMed]
- Parsa AA, Jackowe DJ, Johnson EW, et al. Selection criteria for expander/implant breast reconstruction following radiation therapy. Hawaii Med J 2009;68:66-8. [PubMed]
- Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg 2011;127:2154-66. [PubMed]
- Nahabedian MY. Discussion: Immediate free flap reconstruction of advanced-stage breast cancer: is it safe? Plast Reconstr Surg 2011;128:42-3. [PubMed]
- Nahabedian MY, Momen B. The impact of breast reconstruction on the oncologic efficacy of radiation therapy. Ann Plast Surg 2008;60:244-50. [PubMed]
- Whelan TJ, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol 2011;29: abstr LBA1003.
- Classen J, Nitzsche S, Wallwiener D, et al. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. A retrospective analysis in 109 patients. Strahlenther Onkol 2010;186:630-6. [PubMed]
- Wong JS, Ho AY, Kaelin CM, et al. Incidence of major corrective surgery after post-mastectomy breast reconstruction and radiation therapy. Breast J 2008;14:49-54. [PubMed]
- Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg 2009;33:695-700. [PubMed]
- Serra-Renom JM, Muñoz-Olmo JL, Serra-Mestre JM. Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plast Reconstr Surg 2010;125:12-8. [PubMed]
- Losken A, Pinell XA, Sikoro K, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg 2011;66:518-22. [PubMed]