
A population-based analysis of adenosquamous carcinoma of the salivary gland
Introduction
Adenosquamous carcinoma (ASC) is a malignant epithelial neoplasm characterized by the presence of two malignant components (adenocarcinoma and squamous cell carcinoma) in variable proportions. The primary site of ASC is any organ with a glandular epithelium, such as the gastrointestinal tract organs, cervix, lungs, pancreas, and breasts (1-5). However, this disease rarely occurs in the oral cavity. Originally, consideration of oral ASC as a distinct entity was controversial because this disease was similar to high-grade mucoepidermoid carcinoma (MEC). Compared with MEC, ASC has been shown to be more aggressive, have a worse prognosis, higher metastatic capacity, and pathological differences in few prior case reports. Thus, oral ASC has been considered as a separate and distinct entity of oral malignancies (6-8). The poor prognosis of oral ASC is associated with its aggressiveness, infiltrative nature, local invasiveness, early metastasis, and high recurrence rate after treatment. The optimal treatment is surgery due to its aggressiveness. Meanwhile, the use of radiation therapy and chemotherapy in oral ASC is questionable (9-11).
ASC of the salivary gland is a rare malignancy of the salivary gland like oral ASC. Therefore, there are only few published case reports or case series on the clinical characteristics and prognosis of ASC of the salivary gland (12-14). Kusafuka and Chen described ASC arising from the parotid gland in a 78-year-old woman and 61-year-old man, respectively. The previously described cases showed an aggressive course, with 60% of the patients dying of the disease (15). The clinicopathological characteristics and prognosis of this rare disease remain unclear due to the scarcity of reports. To address this issue, we performed a descriptive, retrospective analysis of patients with oral ASC using data from the Surveillance, Epidemiology, and End Results (SEER) database. The purpose of this report was to assess the clinicopathological characteristics of patients with ASC of the salivary gland and to further characterize survival outcomes in these patients and the effects of different prognostic factors on survival.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-675).
Methods
Data source and participants
The SEER database is a population-based cancer registry maintained by the National Cancer Institute in the United States. It is also an open-access resource containing clinical information on patients with cancer, which currently covers approximately 34.6% of the United States’ population. The database “SEER 18 Regs Custom Data with additional treatment fields, Nov 2018 Sub (1975–2016)” was searched for all patients with ASC of the oral cavity using the SEER*STAT 8.3.6 software (http://www.seer.cancer.gov/seerstat). The third edition of the International Classification of Diseases for Oncology was used to identify all patients diagnosed with this disease using the topography codes of “8982:3” and with the salivary glands as primary sites. The present study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Variables and survival time
Collected variables included demographic information (age at diagnosis, race, sex, laterality); clinicopathological factors [primary tumor site, histology grade, SEER historic stage classification, tumor-node-metastasis (TNM) stage (sixth edition of American Joint Committee on Cancer, AJCC staging system)], treatment (surgery, radiotherapy); and prognosis (survival status and survival in months). Patients were excluded if their follow-up or survival data was unavailable. Overall survival (OS) was the interval from initial diagnosis to death from any cause or last follow-up, and disease-specific survival (DSS) was the interval from initial diagnosis to death from the primary tumor.
Statistical analyses
Categorical data and continuous data are presented as counts with percentages and mean ± standard deviation values (or medians and interquartile ranges (IQR), respectively. Survival probabilities were evaluated using the Kaplan-Meier method, and the log-rank test was used to assess any significant differences in survival stratified by each covariate. To identify statistically significant covariates associated with survival among patients with ASC of the salivary gland, Cox proportional-hazards models were used. A multivariate Cox regression model was used to identify independent prognostic factors and estimate the predictive factors and their weights. Only variables that were significantly associated with survival in the univariate Cox analysis were included in the multivariate Cox analysis. Data were analyzed using IBM SPSS Statistics, version 24.0 (IBM Corp., Armonk, N.Y., USA), and P<0.05 was considered statistically significant.
Results
Clinicopathological characteristics
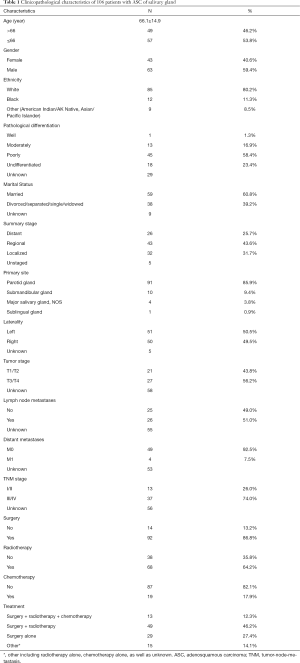
A total of 106 patients with ASC of the salivary gland were identified in the SEER database between 1975 and 2016 (Table 1). The mean age at diagnosis was 66.1±14.9 (range: 17–91) years. There were 63 male patients and 43 female patients, with a male-to-female ratio of 1.47. The most common primary site was the parotid gland (N=91; 85.8%), followed by the submandibular gland (N=10; 9.4%). Of 172 patients with TNM stage information, 13 and 37 patients had stage I/II and III/IV tumors, respectively (AJCC T, seventh edition (2010–2015)]. As defined by the SEER historic stage, the most frequent stage was regional, followed by localized and distant stage. With respect to pathological differentiation, there was a propensity for poorly differentiated tumors, followed by undifferentiated and moderately differentiated tumors. There was no distant metastasis in 91.8% (45/49) of the patients, while 51.0% (26/51) of the patients had nodal involvement. Surgical resection was the primary treatment modality (91/106). Most patients (62/106) underwent surgery followed by radiotherapy, and 19 patients underwent surgery followed by chemoradiotherapy.
Full table
Survival analysis
Kaplan-Meier analysis was performed to estimate OS and DSS. The median OS among all patients was 30 months (95% confidence interval: 19–62). The 1-, 2-, and 5-year OS rates were 71.5%, 55.0%, 41.5%, respectively. The 1-, 2-, and 5-year DSS rates were 80.8%, 72.2%, and 59.2%, respectively (Figure 1A,B). The survival curves of OS and DSS among patients with ASC of the salivary gland stratified according to each stage (SEER historic stage classification and AJCC-TNM stage) are presented in Figure 1. The OS and DSS shortened with increasing tumor stage, regardless of the AJCC TNM stage or SEER historic stage. Compared with patients with localized disease, those with distant or regional disease had a significantly worse prognosis (P<0.01, Figure 1C,D). Similarly, patients with advanced-stage disease (III/IV) had a shorter OS and DSS than those with early-stage disease (I/II) (P<0.01, Figure 1E,F).
Effect of different treatments on prognosis
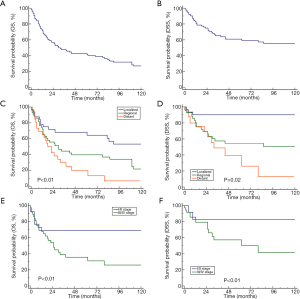
Most of the patients (91/106) underwent surgery. Regardless of the administration of radiotherapy/chemotherapy, the mean OS and DSS were significantly better in the patients who underwent surgery than in those who did not [mOS: 44 vs. 8 months, P<0.01; mDSS: NR (not reached) vs. 9 months] (Figure 2A,B).
After OS stratification according to the surgery-based therapeutic modalities among these patients, the best prognosis was observed in the patients who were treated with a combination of surgery and radiotherapy, followed by those treated with a combination of surgery and chemoradiotherapy, and lastly, those treated with surgery alone (P=0.002) (Figure 2C). The same trend was observed after DSS stratification according to the surgery-based therapeutic modalities; however, no significant difference was observed (P=0.08) (Figure 2D).
Univariate and multivariate Cox survival analyses
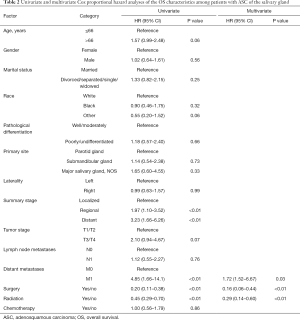
Table 2 and Table 3 show the variables that could influence OS and DSS in Cox survival analysis. The univariate Cox survival analysis showed that distant metastasis and the use of surgery or radiotherapy were significantly associated with OS (P<0.05, Table 2). The multivariate Cox survival analysis demonstrated that only the use of surgery or radiation was an independent prognostic factor for a favorable OS among patients with ASC of the salivary gland (Table 2).
Full table

Full table
While the univariate Cox survival analysis showed that tumor stage and the use of surgery alone were significantly associated with DSS (Table 3), the multivariate Cox survival analysis demonstrated that early stage (T1/T2) and the use of surgery were independent prognostic factors for favorable DSS among the patients with ASC of the salivary gland (Table 3).
Discussion
ASC of the salivary gland is an uncommon malignant tumor, and the clinical characteristics and prognosis of this disease are unclear. Existing information on ASC of the salivary gland is mainly based on case reports and small case series, with limited statistical power (12-14). Thus, an analysis of large databases, such as the SEER database, will provide a more comprehensive and larger sample size cohort of this rare malignancy (16-18). Therefore, this study used data from 106 patients with ASC of the salivary gland obtained from the SEER database to determine the clinicopathologic characteristics, survival, and prognostic factors associated with OS and DSS of this rare disease.
A previous study demonstrated that ASC of the head and neck regions occurs mostly in the tongue, oral floor, and larynx, with a peak in the fifth decade of life and a male-to-female ratio of 3:1 (13). Among the patients with ASC of the salivary gland in this study, the patients were adults, with an average age of 66.1 years (range: 17–91), and no pediatric cases were found, suggesting that ASC of the salivary gland mainly occurs in adults, especially the elderly. We observed a male predominance as shown by a male-to-female ratio of 1.47. Consistent with previous studies (12-14), our study also showed that the parotid gland was the most common primary site (86%, 91/106).
ASC of the salivary gland also exhibited a propensity for regional lymph node metastasis, with 50.9% of patients (26/51) having lymph node metastases. Consequently, most patients with ASC of the salivary gland present with localized or regional disease at diagnosis. For example, 68.3% of the patients had regional-stage or distant-stage disease according to the SEER historic stage classification, and 74.0% of the patients had advanced-stage (III/IV) disease according to the seventh edition of the AJCC staging system. In this study, distant metastases were uncommon (7.5%, 4/53). Thus, early examination and early diagnosis will help shorten the time between onset of nonspecific symptoms to a definitive diagnosis; this is vital in improving the prognosis.
Surgical resection was the primary treatment modality (86.8%, 91/106). Surgery prolonged DSS and OS among patients with ASC of the salivary gland. The multivariate Cox analysis showed that the use of surgery was an independent favorable prognostic factor for DSS and OS. Most patients also underwent radiotherapy (68/106), and 62 patients underwent radiotherapy after surgery. The survival analysis demonstrated that the DSS and OS was better in patients who underwent postoperative radiotherapy than in patients who underwent only surgery. Thus, the combination of surgery and postoperative radiotherapy may be the optimal treatment choice for ASC of the salivary gland. Nineteen patients underwent a combination of surgery and chemoradiotherapy, although DSS and OS were not prolonged by chemotherapy as seen in the survival analysis. The Cox regression analysis also did not reveal a positive association between chemotherapy and prognosis, regardless of OS or DSS.
The prognostic reports on ASC of the salivary gland are generally limited, and the prognosis of this disease remains unclear. Unlike ASC in other sites, our data showed that patients with ASC of the salivary gland had a relatively better prognosis (19-22). For example, the 5-year DSS and OS rates were 59.2% and 41.5%, respectively. However, if patients had regional or distant metastasis, the prognosis was poor. For example, the patients with regional- and distant-stage disease at diagnosis had an mOS of 30.0 and 18 months, respectively, and 5-year OS rates of 36.7% and 13.2%, respectively. The patients with lymph node involvement had a 5-year OS rate of 36.7%. The multivariate Cox regression analysis showed that the presence of distant metastases was an independent prognostic factor for OS, and advanced stage (T3/T4) contributed independently to the shorter DSS among patients with ASC of the salivary gland. In addition, the survival analysis did not find a significant association between OS or DSS and other clinicopathological characteristics. Of course, these associations need to be validated in a larger cohort.
Because of its rarity, a population-based analysis is important for a better statistical power and greater generalizability. Although this study included the largest cohort of patients with ASC of the salivary gland to date, some limitations which are common to studies using the SEER database should be clarified for accurate interpretation of the results (23). First, much information on lymph node metastases, distant metastases, and TNM stage was lacking in 55, 53, and 56 patients, respectively. Although the SEER summary stage has standardized and simplified staging to ensure consistent definitions over time as an alternative to the AJCC stage in the SEER database, there were still five patients without the SEER summary stage. In addition, 29 patients had no information on pathological grade. Some patients had no information on the cause of death in the SEER database, which may have led to the extraction of inaccurate DSSs among the patients. This might explain the discrepancy between the OS and DSS. All these factors limited our ability to describe the reliability and accuracy of our prognostic analysis. Second, other prognostic factors, such as performance status, presence of comorbidities, and resection margin status, were not accessible in the SEER database. Responses to treatment and recurrence rates could not be ascertained from the SEER database. The effect of radiotherapy or chemotherapy alone on survival was not studied because few patients in our cohort underwent radiotherapy or chemotherapy alone. Third, the small sample size with many variables may contribute to the limited statistical power and may cause false-negative findings. Despite these limitations, we believe that the results of this study will provide clinicians with further insights into this rare tumor.
Conclusions
In conclusion, we used a population-based method to present the clinicopathological characteristics and survival analysis of patients with ASC of the salivary gland based on data from the SEER database. This rare malignancy mainly affected adults with a male predominance. Patients with this rare disease had a favorable outcome, with 5-year DSS and OS rates of 59.2% and 41.5%, respectively. Surgery was the main treatment to improve survival, and post-operative radiotherapy could also prolong the OS and DSS. This is the largest case series on ASC of the salivary gland; these results are vital to disease management and future prospective studies of this rare malignancy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-675
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-675). The authors declared no any conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen YY, Li AF, Huang KH, et al. Adenosquamous carcinoma of the stomach and review of the literature. Pathol Oncol Res 2015;21:547-51. [Crossref] [PubMed]
- Hsu JT, Chen HM, Wu RC, et al. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol 2008;6:95. [Crossref] [PubMed]
- Masoomi H, Ziogas A, Lin BS, et al. Population-based evaluation of adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum 2012;55:509-14. [Crossref] [PubMed]
- Soo K, Tan PH. Low-grade adenosquamous carcinoma of the breast. J Clin Pathol 2013;66:506-11. [Crossref] [PubMed]
- Wang J, Wang FW, Lagrange CA, et al. Clinical features and outcomes of 25 patients with primary adenosquamous cell carcinoma of the prostate. Rare Tumors 2010;2:e47. [Crossref] [PubMed]
- Alos L, Castillo M, Nadal A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology 2004;44:570-9. [Crossref] [PubMed]
- Sravya T, Rao GV, Kumar MP, et al. Oral adenosquamous carcinoma: Report of a rare entity with a special insight on its histochemistry. J Oral Maxillofac Pathol 2016;20:548. [Crossref] [PubMed]
- Thanakappan P, Venkata NS, Amudala R, et al. Adenosquamous carcinoma of oral cavity. J Cancer Res Ther 2015;11:1034. [Crossref] [PubMed]
- Damiani JM, Damiani KK, Hauck K, et al. Mucoepidermoid-adenosquamous carcinoma of the larynx and hypopharynx: a report of 21 cases and a review of the literature. Otolaryngol Head Neck Surg 1981;89:235-43. [Crossref] [PubMed]
- Napier SS, Gormely JS, Newlands C, et al. Adenosquamous carcinoma. A rare neoplasm with an aggressive course. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;79:607-11. [Crossref] [PubMed]
- Sheahan P, Fitzgibbon J, Lee G, et al. Adenosquamous carcinoma of the tongue in a 22-year-old female: report of a case with immunohistochemistry. Eur Arch Otorhinolaryngol 2003;260:509-12. [Crossref] [PubMed]
- Chen WH, Pei J, Zhuo XY. Adenosquamous carcinoma of the parotid gland: report of 1 case and review of the literature. Shanghai Kou Qiang Yi Xue 2015;24:763-5. [PubMed]
- Kimihide K, Tomoko M, Takashi N. Adenosquamous carcinoma of the parotid gland. Histopathology 2013;63:593-5. [Crossref] [PubMed]
- Kusafuka K, Miki T, Nakajima T. Adenosquamous carcinoma of the parotid gland. Histopathology 2013;63:593-5. [Crossref] [PubMed]
- Scully C, Porter SR, Speight PM, et al. Adenosquamous carcinoma of the mouth: a rare variant of squamous cell carcinoma. Int J Oral Maxillofac Surg 1999;28:125-8. [Crossref] [PubMed]
- Dubal PM, Unsal AA, Chung SY, et al. Population-based trends in outcomes in adenoid cystic carcinoma of the oral cavity. Am J Otolaryngol 2016;37:398-406. [Crossref] [PubMed]
- Lin F, Duan J, Lin Y, et al. Survival and risk factors in patients with liposarcoma with distant metastasis. Am J Transl Res 2020;12:2071-82. [PubMed]
- Wang Y, Wang S, Zhang B. A Population-Based Analysis of Mucoepidermoid Carcinoma of the Oral Cavity. Laryngoscope 2021;131:E857-63. [Crossref] [PubMed]
- Qin BD, Jiao XD, Yuan LY, et al. Adenosquamous carcinoma of the bile duct: a population-based study. Cancer Manag Res 2018;10:439-46. [Crossref] [PubMed]
- Wang J, Lian B, Ye L, et al. Clinicopathological characteristics and survival outcomes in adenosquamous carcinoma of the lung: a population-based study from the SEER database. Oncotarget 2018;9:8133-46. [Crossref] [PubMed]
- Ge Y, Lin L, Ma X, et al. Adenosquamous Carcinoma of the Stomach: A Population-based Study from the SEER Database. J Cancer 2019;10:5705-13. [Crossref] [PubMed]
- Yendamuri S, Malhotra U, Hennon M, et al. Clinical characteristics of adenosquamous esophageal carcinoma. J Gastrointest Oncol 2017;8:89-95. [Crossref] [PubMed]
- Bai J, Zhao F, Pan S. Clinicopathological Characteristics and Survival of Small Cell Carcinoma of the Salivary Gland: a Population-Based Study. Cancer Manag Res 2019;11:10749-57. [Crossref] [PubMed]


