Minimally invasive parathyroid surgery
Background
Historically, until the 1990s, bilateral cervical exploration for localization of all four parathyroid glands and removal of any that were grossly enlarged had been the standard surgical treatment for primary hyperparathyroidism (PHPT). Challenges with intraoperative adenoma localization and damage to surrounding structures have long hindered utilizing less invasive approaches for parathyroidectomy. In the past two decades however, significant improvements in the accuracy and reliability of preoperative localization studies have facilitated further advances in surgical management, allowing a more targeted, minimally invasive surgical approach. Minimally invasive parathyroidectomy (MIP) is defined as any focused surgical approach that preoperatively aims to identify and remove a single enlarged parathyroid gland (focused or targeted parathyroidectomy) and may in certain circumstances allow examination of the ipsilateral gland as well (unilateral parathyroidectomy). Because 80-90% of patients with PHPT have a solitary parathyroid adenoma, resection of one gland leads to cure (normocalcemia for 6 months following surgery) in most cases while eliminating the unnecessary dissection of multiple glands or a bilateral exploration.
The first unilateral approach for solitary parathyroid adenomas was reported in 1982. The procedure involved the removal of both a parathyroid adenoma and ipsilateral normal parathyroid gland (1). Since then, several minimally invasive techniques have been described, including radio-guided parathyroidectomy, endoscopic parathyroidectomy with gas insufflation, and video-assisted parathyroidectomy without gas insufflation. The confluence of improved adenoma localization using different preoperative localization studies and the concomitant advent of minimally invasive approaches have led to fewer complications, shorter operative time, shorter hospitalization, a more rapid postoperative recovery, an improved cosmetic result, and greater patient satisfaction (2,3). Minimally invasive parathyroidectomy has become the preferred procedure over bilateral neck exploration for PHPT by most endocrine surgeons. Nonetheless, a prospective randomized controlled trial, conducted by a large volume center with significant experience comparing unilateral to bilateral neck exploration showed no statistical differences between complication rates, costs, and operative time between the two groups (4). If this experience is not available, MIP is recommended when a parathyroid adenoma is localized preoperatively, as it can be removed without visualizing the other glands, and the rapid intraoperative parathyroid hormone (IOPTH) assay is employed to confirm an adequate resection. Minimal access techniques have therefore replaced a bilateral neck exploration in patients with localized disease, although a traditional cervical incision with bilateral neck exploration remains the indicated approach for non-localized disease.
Candidates for minimally invasive parathyroidectomy
Patients being evaluated for MIP should undergo a comprehensive history and physical examination in addition to routine laboratory testing to establish the diagnosis of PHPT. While most patients with PHPT are asymptomatic, this does not negate the need for eliciting pertinent symptoms related to a history of renal calculi, renal failure, pathologic fractures, and a family history of multiple endocrine neoplasia (MEN). In addition to this, a history of prior neck surgery should be obtained. A physical examination should be performed and should include palpation for existing thyroid nodules or lymphadenopathy. The next step in the workup of a patient with a presumed hyperfunctioning parathyroid gland should include efforts to definitely localize the gland, which entails the use of high-resolution radiographic imaging and nuclear medicine modalities.
The indications for MIP are the same as those for traditional cervical exploration, that is, symptomatic patients or those with asymptomatic PHPT fulfilling the criteria established by the most recent National Institutes of Health consensus meeting (Table 1) (5). Consideration should be given to the differential diagnosis of benign familial hypocalciuric hypercalcemia and vitamin D deficiency based on past medical history and interpretation of biochemical values. A MIP technique can be successfully performed for patients with persistent or recurrent disease. However, MIP is rarely employed when preoperative localization of the hyperfunctioning parathyroid gland is not performed, is negative, discordant on multiple imaging studies, or is consistent with multiglandular enlargement. A conventional, 4-gland exploration approach should be performed in these cases and is usually accomplished through a small cervical incision. A comprehensive list of contraindications to MIP is presented in Table 2.

Full table

Full table
The informed consent process should include discussion with the patient on the possibility of conversion to a traditional incision or 4-gland exploration around 7% of the time based on data from large-volume centers, in addition to the usual risks of parathyroid surgery.
Anatomic considerations
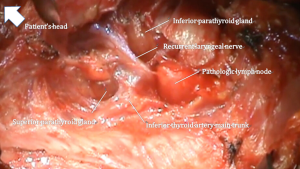
The focused approach of MIP requires an intimate familiarity with the location of normal and aberrant parathyroid glands and their relationship to surrounding landmarks within the neck. The location of the parathyroid gland follows definite embryonically influenced patterns. Superior parathyroid glands are embryologically derived from the 4th branchial pouch while the inferior parathyroid glands originate from the 3rd branchial pouch. As superior parathyroid glands have a shorter distance of migration, their location tends to be more consistent compared to that of the inferior parathyroid glands. A systematic approach should be adopted in obtaining adequate exposure for visualization of the parathyroid bearing regions which often entails ligation of the middle thyroid vein to allow for medial retraction of the thyroid lobe, identification of the prevertebral fascial plane (also known as the viscerovertebral angle) between the thyroid lobe and carotid artery and the tracheoesophageal groove. Following medial retraction of the thyroid lobe, the superior parathyroid glands are usually located within the tracheoesophageal groove and have a posterior-lateral relationship to the recurrent laryngeal nerve. Aberrant superior parathyroid glands may however lie within the posterior superior mediastinum or in a retroesophageal location. The inferior parathyroid artery commonly courses anteriorly to the gland. Inferior parathyroid adenomas usually lie in an antero-inferior location to the thyroid lobe within a fatty envelope, which is often contiguous with the thyrothymic ligament. The recurrent laryngeal nerve conventionally has a posterior relation to the inferior parathyroid gland and once identified, dissection of the gland proceeds first along its lateral and posterior aspect to allow for progressive medial retraction and eventual ligation of medial vessels close to the thyroid gland (Figure 1). The absence of an inferior parathyroid gland in its normal location should prompt exploration in a perithymic location or superior anterior mediastinum. A summary of anatomic pearls for MIP is presented in Table 3.

Full table
Preoperative localizing imaging studies
Although preoperative localizing studies were historically considered not necessary for patients undergoing initial bilateral neck exploration, current trends toward minimally invasive surgery with limited exploration of the neck are only made possible by preoperative localization studies. Parathyroid localizing studies have evolved since their inception in the late 1960s. Today, the role of preoperative localization studies is to assist the surgeon in identifying the precise anatomic location of a hyperfunctioning parathyroid gland and its relationship to adjacent structures, thus enabling a more focused exploration. Imaging studies have no role in the diagnosis PHPT or in the decision to proceed with surgery. Preoperative localization studies should only be obtained after a diagnosis of PHPT is made. Presently a variety of noninvasive and invasive imaging studies are available. Usually, preoperative imaging studies include ultrasound, nuclear medicine (specifically the sestamibi scan), or computed tomography examinations, either alone or in combination. Ideally, preoperative localization studies should include a nuclear medicine imaging study to provide information of gland function in combination with an anatomic imaging study, to provide useful information about surrounding structures and regional anatomy. This information, gathered by combination of both imaging modalities, allows for optimization of preoperative planning and surgery.
The quality, sensitivity, and specificity of these imaging studies depend on the skill and experience of the person performing and interpreting them, therefore, it is necessary to secure the services of a dedicated team of imaging professionals with experience and an interest in parathyroid disease. The surgeon should direct the selection and order of the preoperative images to be obtained in order to tailor the most efficient and effective surgical intervention.
Noninvasive methods
High-resolution ultrasound
Of all the imaging modalities, ultrasound is the least expensive and least invasive; it does not involve radiation and is readily accessible. It is performed using a high-frequency linear transducer ideally in the range of 12-15 mHz. Parathyroid glands appear as well-circumscribed and oval, hypoechoic, and usually solid nodules. Table 4 summarizes the factors that limit the accuracy of ultrasound imaging. The surgeon must always keep in mind the possibility of an intrathyroidal parathyroid adenoma, which can present in up to 5% of cases, thus requiring proper patient counseling for a possible thyroid lobectomy. The sensitivity of ultrasound detection of parathyroid adenomas ranges from 27-95%, with a specificity of 92-97%. It is the operator experience that has the greatest effect on the ability to detect diseased parathyroid glands and likely accounts for the wide range of reported sensitivity.

Full table
US-guided fine-needle aspiration (FNA) can be considered to confirm intrathyroidal parathyroid adenomas or in selected cases of persistent or recurrent PHPT after failed exploration. An elevated PTH washout concentration from the FNA can help identify parathyroid gland lesions. With the PTH washout technique, MIP can be implemented even with negative cytology, thus allowing success of a targeted surgical approach in difficult reoperative cases.
Nuclear medicine sestamibi scan
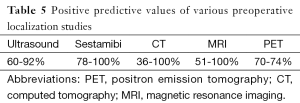
Technetium (Tc-99m) sestamibi scan detects mitochondrial uptake of the radionuclide tracer in areas of hyperfunctioning tissue. The injected tracer is initially concentrated in normal thyroid and abnormal parathyroid tissues. The concentration in normal thyroid tissue decreases rapidly, leaving behind foci of relatively enhanced uptake of the tracer in abnormal thyroid and parathyroid tissues. After injection of the radiotracer, one set of images is taken within 15 minutes and then a delayed set is taken at 2 hours. Asymmetry of uptake can be noted on early images, but usually, the delayed images are necessary to locate the focus of radiotracer, which characterizes hyperfunctioning parathyroid. A lack of retention of the tracer does not exclude the diagnosis of PHPT, as sestamibi imaging can miss small parathyroid adenomas and hyperplasia. The combination of ultrasound and sestamibi scintigraphy to localize hyperfunctioning parathyroid glands preoperatively increases the sensitivity to 95% because each modality contributes complementary data to help determine the offending gland(s) location (Table 5) (6).
Full table
Multiphase computed tomography (CT)
This is an imaging modality that is similar to CT angiography. The name is derived from 3-dimensional CT scanning with an added dimension from the changes in perfusion of contrast over time. Multiphase CT generates exquisitely detailed, multilane images of the neck and allows the visualization of differences in the perfusion characteristics of hyperfunctioning parathyroid glands (i.e., rapid uptake and washout), compared with normal parathyroid glands and other structures in the neck. The images that are generated by multiphase CT provide both anatomic information and functional information in a single study that the operating surgeon can interpret easily and may serve an important role in localization before both initial and reoperative parathyroid procedures.
Magnetic resonance imaging (MRI)
MRI may be selectively applied for parathyroid imaging. Hyperfunctional parathyroid glands tend to be isointense to low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and with intense enhancement after intravenous gadolinium administration. MRI evaluation of parathyroid localization may be more applicable for ectopic adenomas located in the mediastinum. Limitations of the use of MRI include cost and patient compliance with reference to a sense of close confinement during examination.
Invasive methods
Fine-needle aspiration (FNA) biopsy
It may be applied with either CT or ultrasound guidance for correct needle placement in suspected abnormal parathyroid tissue during localization in preparation for reoperative surgery, where radiographic findings are otherwise equivocal. Cytologic evaluation of tissue samples obtained by FNA biopsy is less sensitive than measuring washout PTH levels of the aspirate material, because follicular thyroid tumors may be misinterpreted as parathyroid tissue under cytologic review. PTH washout is an accurate way to localize culprit lesions in patients with findings indicating parathyroid lesions on neck ultrasound.
Selective arteriography
Selective angiographic injection of the inferior thyroid arteries will demonstrate a vascular blush that may be present in up to 25-70% of parathyroid adenomas. Significant complications attributable to this technique have been reported and include central nervous system embolic infarction and potential quadriplegia. As a consequence of these potential risks and because of improvements in noninvasive imaging studies, selective parathyroid arteriography is rarely performed currently. A more selective arteriographic modality is that of super selective digital subtraction angiography. This is a highly sensitive method for localization of ectopic parathyroid tissue and has an indication for patients with recurrent or persistent PHPT in whom previous noninvasive testing has failed to identify the adenoma in a usual or utopic location.
Selective venous sampling
This modality is performed by catheterization of veins draining the neck and mediastinum. By obtaining blood samples and comparing PTH levels obtained from sampling of the iliac veins with those obtained from thyroid veins (superior, middle and inferior), vertebral veins, and the thymic vein, the anticipated location of the adenoma will be within the area where venous PTH levels are at least twice as high as the systemic levels. Selective venous sampling has been shown to be more accurate than large vein sampling, with accuracy of 83% as contrasted to 29%, respectively. This modality became significantly more accurate with the utilization of improved intact PTH assays, which increased the sensitivity of venous sampling to 87-95% in some investigations. Selective venous sampling should be reserved for patients requiring reoperation and in whom noninvasive studies are negative, equivocal, or conflicting. This modality is technically challenging and its success depends on an experienced interventional radiologist.
Intraoperative adjuncts
Intraoperative ultrasound
The availability of high-resolution ultrasound has led some surgeons to further utilize it in their operating room. Intraoperative ultrasound may be useful in a number of operative settings. Using this adjunct will allow the surgeon to scan the neck and, where possible, correlate structures with preoperative images just prior to surgery. This achieves accurate visualization of the ultimate position of both the parathyroid lesion and other structures in the neck, in particular the relation to the internal jugular vein and carotid artery. This technique may also assist in precisely localizing the incision once the patient is in the neck extension position, for an ideal access for removal of parathyroid tissue. Ultrasound can be combined with FNA for PTH to interrogate hypoechoic structures identified in the thyroid or neck intraoperatively.
Frozen section analysis
The histological identification of parathyroid tissue relies on the identification of three types of cells that comprise the parathyroid tissue; chief, oxyphil, and water clear cells. Chief cells can be similar to thyroid follicular cells and oxyphil cells are indistinguishable from thyroid Hurthle cells, thus the distinction of parathyroid from thyroid tissue is more challenging. However, follicles and colloid like material are uncommon in parathyroid specimens. Frozen section is an unreliable method for distinguishing between multiglandular disease and adenomas. The distinction between hyperplasia and adenoma is not based on pathologic criteria, rather on the operative findings. If the pathologist receives a biopsy from a single parathyroid gland for frozen section interpretation, the possible diagnosis would be, hypercellular parathyroid tissue. An adenoma can be diagnosed with confidence if only one gland of the four glands is enlarged and hypercellular. Therefore, without biopsies from all four glands, the pathologist is unable to determine the cellularity of the remaining parathyroid glands. Nevertheless, the use of frozen section to distinguish parathyroid tissue from non-parathyroid tissue has an accuracy of 99.2% (7).
Intraoperative PTH assay
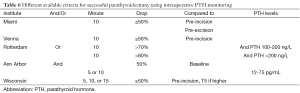
To further improve the surgical success of MIP and to minimize the possibility of persistent or recurrent PHPT after surgery, some have advocated the use of surgical adjuncts such as IOPTH monitoring. IOPTH is useful in assessing the adequacy of resection by functional means without the need to expose all the parathyroid glands. Before exiting the operating room, the surgeon can confirm that the patient will be eucalcemic by demonstrating an appropriate reduction in IOPTH levels after excision of all hyperfunctioning parathyroid tissue. The ability to confirm complete removal of all hypersecreting glands and predict operative success minimizes operative time, diminishes the need for bilateral neck exploration, and improves cure rates (8). IOPTH is based on the short half-life of circulating PTH. PTH is cleared from the blood in an early rapid phase with a half-life variably reported as 1.5-21.5 minutes in patients with normal renal function. PTH levels are measured preoperatively and at set post-excision times. Due to the different IOPTH decrease criteria for a successful operation, several studies aimed to identify the optimal criteria and its predictive cure rate. A decline of >50% in iPTH level from the highest pre-incision or pre-excision level is associated with predictive cure in 94-97% of cases (9,10). We prefer a PTH drop of at least 50% and into the normal range before concluding the procedure. The single criterion of PTH drop into the normal range is somewhat problematic as some patients have a normal or slightly elevated baseline levels and thus some institutions adjusted their criteria and required a 50% PTH drop with and/or normalization of PTH levels. Different criteria may be utilized with similar accuracy rates (Table 6) (11). When used correctly, we believe that IOPTH is the most accurate adjunct available to the surgeon performing parathyroid surgery. Nonetheless, when there are concordant Sestamibi scans and US localization, IOPTH appears to add little benefit to the cure rate (12).
Full table
Of note, there is a distinct entity of parathyroid disease that manifests normal PTH levels and can therefore be referred to as normohormonal PHPT. Interestingly, a normohormonal parathyroid disorder may occur with iPTH levels as low as 5-15 pg/mL, which is at the lowest reference point of most iPTH assays or below the detectable range. Awareness of this unusual phenotype may facilitate earlier diagnosis and surgery. Nonetheless, it is particularly difficult to apply this intraoperative adjunct to decide the surgical cure of the patient when the serum PTH level is within the normal range. Very few studies have focused on this issue, and we believe the utility of IOPTH monitoring in this subset of patients needs to be further studied.
Surgical technique
Minimally invasive parathyroidectomy encompasses a number of different techniques, including open approaches using “mini-incisions”, minimally invasive radio-guided parathyroidectomy, video-assisted parathyroidectomy, and purely endoscopic parathyroidectomy with or without robotic assistance. Nonetheless, the term minimally invasive should be reserved for procedures that allow parathyroidectomy through access that minimizes trauma of the surgical exposure and dissection. Minimally invasive parathyroidectomy should obtain at least the same cure outcome of traditional 4-gland exploration, with the main advantage of reducing the skin incision and, consequently, allowing better cosmetic results. Other advantages are decreased postoperative pain, which should be mainly related to less extensive surgical dissection.
Open minimally invasive parathyroidectomy
This approach represents the most widespread minimally invasive technique, performed through a small, 1-2 inch central, or a lateral (over the adenoma location, overlying the anterior border of the sternocleidomastoid muscle) incision, guided by preoperative localization studies and IOPTH. This approach is straightforward, can be reproduced in different surgical settings, and can be performed under locoregional anesthesia, with reduced operative time and as a short-stay procedure (13).
Using a central or midline incision allows access to virtually all localized inferior glands and many non-ectopic superior parathyroid glands. The lateral approach is classically used for re-operative parathyroid surgery. Some advocate this approach for superior parathyroid adenomas that are deep or lateral in their position. Regardless of the type of approach, general surgical principles similar to that of traditional parathyroidectomy are of great importance, given the narrow working space. These include meticulous hemostasis with clips or diathermy, adequate exposure of the prevertebral fascia between the thyroid lobe and carotid artery, and the identification and preservation of the recurrent laryngeal nerve. Once the parathyroid tumor is identified, it is highly critical to handle the tumor gently to avoid rupture of the capsule with ensuing spillage and bleeding.
The main limitation of this technique resides in the potential poor visualization of the neck structures, because of the small size of the skin incision or, conversely, the need for a larger skin incision when compared with video-assisted or endoscopic techniques. An advantage is quick conversion to a bilateral neck exploration if need be, sometimes even without enlargement of the skin incision.
Video-assisted parathyroidectomy
This approach was first described by Miccoli et al. (14). Early after its first description, this technique encountered worldwide acceptance, as it is easy to reproduce in different surgical settings. This method requires two assistants, one for holding retraction and the other for endoscope placement in addition to the primary surgeon, with monitors placed on either side of the assistants for optimal visualization of the surgical field. A 1.5 cm transverse incision is carried out 2 cm above the sternal notch, followed by dissection to the level of the midline raphe. The midline raphe is divided in a superior to inferior direction by 4 cm, followed by retraction of the strap muscles ipsilateral to the localized adenoma. A second retractor is utilized to perform medial reflection of the ipsilateral thyroid lobe. Insertion of a 30° 5 mm endoscope through the insertion follows. Exploration commences on the ipsilateral side of the anticipated location of the parathyroid adenoma based on preoperative imaging. The middle thyroid vein is divided, followed by the use of a dissecting spatula to expose the carotid artery laterally, pre-vertebral plane posteriorly and groove between the thyroid and trachea using spatulated instruments before surgical exploration of the parathyroid adenoma commences. Effort is made to identify the location and course of the recurrent laryngeal nerve before any vessel ligation around the vicinity of the parathyroid adenoma is performed. The hyperfunctioning parathyroid gland is then removed and retrieved through the incision followed by measurement of IOPTH prior to closure of the incision site.
The main advantages of these approaches are the direct access to the gland and small neck incisions. To date, all published series reported less operative pain, better cosmesis, shorter hospital stay, and ability to perform these surgeries on an outpatient basis. However, since incisions are made in the neck, for some they remain visible and prone to hypertrophy and keloid formation. Furthermore, some surgeons would argue that given the strict selection criteria used, such as nodule size ≤3 cm in diameter, thyroid volume ≤30 mL, previous conventional neck surgery, recurrent or persistent PHPT, an open approach using a similar incision is also possible. This partly led some surgeons to pursue the indirect/extracervical, remote approaches. Nonetheless, with increasing experience, selection criteria for minimally invasive video-assisted parathyroidectomy have been refined and widened.
Radio-guided Tc-99m sestamibi
Tc-99m sestamibi uptake by parathyroid tissue is a function of metabolic activity. This forms the basis for utilizing sestamibi scans to localize hyperfunctioning parathyroid glands in patients with PHPT. Patients are injected with a Tc-99m sestamibi isotope on the day of surgery, usually within approximately 2 hours of the operation. A hand-held gamma probe is used to direct the incision site and to localize the abnormal parathyroid glands. The initial scan provides information regarding localization of presumed adenomas and the presence of delayed uptake of nuclear material within the thyroid gland. After identification and removal of the abnormal parathyroid gland, the gamma probe may be used to confirm high metabolic activity within the resected tissue as compared with the radioactivity of the surgical bed, thus validating that no additional hyperactive glands remain behind. Potential advantages of radioguided parathyroid identification include facilitation of targeted parathyroidectomy, shorter operating time and verification of successful surgery. Absolute contraindications for radioguided parathyroidectomy include pregnancy and allergy or sensitivity to Tc-99m sestamibi. The Twenty percent rule, published by Murphy and Norman, which suggests that any excised tissue containing >20% of background radioactivity in a patient with a positive Sestamibi scan, results in finding a solitary parathyroid adenoma (15). This protocol, however, has limited ability to exclude non parathyroid tissue or multiglandular disease, and that the signal is proportional to the gland size.
The overall accuracy of radioguided parathyroidectomy is 83% with a conversion rate to bilateral neck exploration of 10% for single gland disease, 50% for multiglandular disease and 50% for hyperplasia. The gamma probe is considered unhelpful in up to 48% of cases. The limitations include logistic difficulties with timing isotope injection, equipment problems, confusing counts, and easily identified abnormal glands. Therefore, most parathyroid surgeons will only consider intraoperative radioguided parathyroidectomy in patients with an ectopic parathyroid adenoma or previous thyroidectomy where confusing background counts are not a concern.
Endoscopic parathyroidectomy
Procedures that utilize the endoscope take advantage not only of a targeted approach but also of the endoscopic magnification with optimal visualization of the neck structures, such as the recurrent laryngeal nerve and parathyroid glands. These procedures require dedicated surgical instrumentation, and an adequate and relatively prolonged learning curve.
Anterior endoscopic approach
Gagner first described this totally endoscopic approach in 1996 (16). It is carried out entirely under steady gas flow, using a 5 mm endoscope introduced through a central trocar, and two or three additional trocars for the instruments. The dissection is first performed beneath the platysma to obtain a good working space. The midline is then opened and the strap muscles retracted to expose the thyroid lobe and explore the parathyroid glands after dissecting the thyroid from the fascia.
These procedures provide an optimal cosmetic result because of the small, distant scars, but they are difficult to reproduce, especially by surgeons who do not have endoscopic experience. In addition, the risks related to CO2 absorption are not completely eliminated.
Lateral endoscopic approach
This approach was first described by Henry et al. (17), characterized by a 12 mm skin incision on the anterior border of the sternocleidomastoid muscle, 3 to 4 cm above the sternal notch on the side of the affected parathyroid gland. Through this incision the tissue is dissected with an open technique in order to reach the prevertebral fascia. Once enough space has been created, two 2.5-mm trocars are inserted on the line of the anterior border of the sternocleidomastoid muscle 3 to 4 cm above and below the first incision through which a 10-mm trocar for the endoscope (10 mm, 0 degrees) is inserted. Dissection is carried out with 8 mmHg carbon dioxide insufflation during the whole procedure. The main technical limitation of the technique is the unilateral approach that prevents the possibility of accomplishing a bilateral exploration when necessary without conversion to an open conventional procedure.
Extracervical/remote access approaches
These approaches involve placing incisions outside the neck, requiring extensive dissection under the skin. The operating space is maintained by either CO2 insufflation or external retraction by specially designed skin retractors. Recently, the application of robotic technology to further assist the surgeon in accomplishing these techniques facilitated remote access parathyroid surgery and helped avoid the need for insufflation (18). The addition of the da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) could make some of the extracervical approaches technically less challenging but certainly adds cost to the procedure. Moreover, it should be emphasized that the remote access approach is not considered “minimally invasive”, as it actually requires much more dissection than the traditional endoscopic parathyroidectomy. A robotic-assisted thoracoscopic approach, an alternative to conventional invasive sternotomy, has also been reported for parathyroid adenomas located within the mediastinum (19).
Summary
The success of MIP has been established by several studies displaying cure and complication rates that are at a minimum equivalent to those achieved by conventional 4-gland exploration. In contrast to bilateral exploration, MIP has been shown to be associated with significantly reduced complications (1.2% vs. 3.1%), enhanced cure rates (99.4% vs. 97.1%), an approximate 50% reduction in operating time (1.3 vs. 2.4 hours), a sevenfold reduction in length of hospital stay (0.24 vs. 1.64 days), and a mean savings of $2,700 per procedure (2,3). A prospective randomized controlled trial comparing unilateral to bilateral neck exploration showed no statistical differences between complication rates, costs, and operative time between the two groups (4).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tibblin S, Bondeson AG, Ljungberg O. Unilateral parathyroidectomy in hyperparathyroidism due to single adenoma. Ann Surg 1982;195:245-52. [PubMed]
- Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 2002;235:665-70. [PubMed]
- Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg 2011;253:585-91. [PubMed]
- Bergenfelz A, Lindblom P, Tibblin S, et al. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: a prospective randomized controlled trial. Ann Surg 2002;236:543-51. [PubMed]
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 2014;99:3561-9. [PubMed]
- Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005;132:359-72. [PubMed]
- Westra WH, Pritchett DD, Udelsman R. Intraoperative confirmation of parathyroid tissue during parathyroid exploration: a retrospective evaluation of the frozen section. Am J Surg Pathol 1998;22:538-44. [PubMed]
- Chen H, Pruhs Z, Starling JR, et al. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery 2005;138:583-7. [PubMed]
- Irvin GL 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay: surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg 2004;28:1287-92. [PubMed]
- Chiu B, Sturgeon C, Angelos P. Which intraoperative parathyroid hormone assay criterion best predicts operative success? A study of 352 consecutive patients. Arch Surg 2006;141:483-7. [PubMed]
- Mazeh H, Chen H. Intraoperative adjuncts for parathyroid surgery. Expert Rev Endocrinol Metab 2011;6:245-53.
- Gawande AA, Monchik JM, Abbruzzese TA, et al. Reassessment of parathyroid hormone monitoring during parathyroidectomy for primary hyperparathyroidism after 2 preoperative localization studies. Arch Surg 2006;141:381-4. [PubMed]
- Lee JA, Inabnet WB 3rd. The surgeon’s armamentarium to the surgical treatment of primary hyperparathyroidism. J Surg Oncol 2005;89:130-5. [PubMed]
- Miccoli P, Pinchera A, Cecchini G, et al. Minimally invasive, video-assisted parathyroid surgery for primary hyperparathyroidism. J Endocrinol Invest 1997;20:429-30. [PubMed]
- Murphy C, Norman J. The 20% rule: a simple, instantaneous radioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery 1999;126:1023-8. [PubMed]
- Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg 1996;83:875. [PubMed]
- Henry JF, Defechereux T, Gramatica L, et al. Minimally invasive videoscopic parathyroidectomy by lateral approach. Langenbecks Arch Surg 1999;384:298-301. [PubMed]
- Noureldine SI, Lewing N, Tufano RP, et al. The role of the robotic-assisted transaxillary gasless approach for the removal of parathyroid adenomas. ORL J Otorhinolaryngol Relat Spec 2014;76:19-24. [PubMed]
- Bodner J, Profanter C, Prommegger R, et al. Mediastinal parathyroidectomy with the da Vinci robot: presentation of a new technique. J Thorac Cardiovasc Surg 2004;127:1831-2. [PubMed]


