Immediate breast volume replacement using a free dermal fat graft after breast cancer surgery: multi-institutional joint research of short-term outcomes in 262 Japanese patients
Introduction
In the 1980s, breast-conserving surgery (BCS) rapidly became the first-line procedure for early-stage breast cancer as it ensured local control and produced acceptable cosmetic results (1-4). Many factors are known to influence cosmetic outcomes (5-7), such as the tumor size and location in the breast parenchyma. Poor cosmetic results are related to larger tumors, especially relative to breast size, and to inner quadrant tumors. A previous study identified the factors influencing cosmetic outcomes after breast-conserving therapy (BCT) for breast cancer and suggested the importance of both tumor-related and treatment-related factors (8). Studies on oncoplastic surgery combining partial mastectomy with immediate volume replacement have been conducted in Japan, and some simple cosmetic techniques for repairing partial defects in various locations can be performed during BCT and have achieved excellent results (9-12). Since 2003, Kijima et al. has reported oncoplastic breast surgery (OBS) combining partial mastectomy and immediate volume replacement using a free dermal fat graft (FDFG). They performed a wide excision for cancer lesions in the upper inner quadrant and immediate reconstruction using FDFG from the lower abdomen with/without axillary lymphadenectomy. The early experiences of this surgical method were reported in detail and it was concluded to be useful for the immediate reconstruction of partial defects during BCT (13). Reconstructions using an autologous FDFG were easy to perform and produced excellent cosmetic results (14). However, guidelines to recommend which quadrant should be repaired by this method, references to the size of FDFG, and how often postoperative complications occur currently do not exist. Therefore, we examined a large multi-institutional data set of patients undergoing partial mastectomy or total mastectomy followed by immediate breast reconstruction using FDFG in order to determine what the risk factors for complications were and identify preoperative clinical factors associated with postoperative outcomes. We hypothesized that factors associated with complications may occur at a frequency that varies according to factors associated with the patient and also the surgeon, in addition to oncological findings such as the tumor location, size of partial mastectomy, as well as the existence of systemic diseases such as diabetes mellitus, a smoking habit, experience of the surgeon, case numbers in one institution, and details for surgery. The purpose of this study is to clarify the cause of postoperative complications after breast cancer surgery with immediate volume replacement using FDFG routinely done in Japan and whether the indication should be determined.
Patients and methods
Patients
A retrospective analysis of a prospectively maintained database of patients undergoing BCS and immediate breast reconstruction using FDFG from 14 hospitals in Japan was performed with Institutional Review Board approval. Operative cases performed between October 2003 and December 2010 comprised the data set for this analysis. All patients underwent radical and curative resections. Fellowship-trained breast surgeons or plastic surgeons performed operative procedures at each institution. Each institution provided specified preoperative, operative, and postoperative data elements using a common menu-derived database file that incorporated precise coding instructions and dropdown menu options where appropriate. Patient selection, surgical procedures such as partial mastectomy (Bp), quadrantectomy (Bq), or total mastectomy (Bt), resection of the fascia of the major pectoralis muscle, and the choice of neo-adjuvant, adjuvant systemic, and postoperative radiation therapies were determined by the individual surgeon. The surgical period was recorded and included the waiting period for a pathological examination of surgical margins and/or sentinel lymph nodes. A data sheet was retrospectively collected as a questionnaire from each institution.
Surgical technique
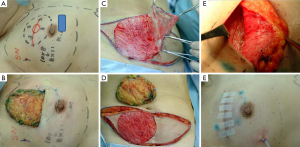
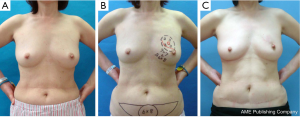
Partial mastectomy or total mastectomy and immediate reconstruction using FDFG were basically carried out using a previously reported method (13). The operative defect was measured pre- and intra-operatively to decide the size of FDFG. Each surgeon/plastic surgeon chose the donor sites of FDFG freely (lower abdomen, lateral abdomen, inguinal region, and femoral region). After in situ de-epithelialization and sharp dissection, FDFG was harvested as a columnar-shaped specimen. Some surgeons used a knife for de-epithelialization, while others used scissors or a dermatome. We intraoperatively measured the defect size of the breast as the horizontal and cranio-caudal lengths and thickness of the resected gland. We trimmed the size of FDFG (horizontal and craniocaudal lengths, and cranial and caudal thicknesses) based on these measured values. FDFG was inserted in an ideal direction that fit the dermis and irregular defects with the dermis facing the surface of the pectoralis major muscle (Figure 1). Details were recorded in Table 1.
Full table
Follow-up
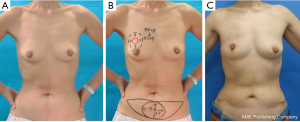
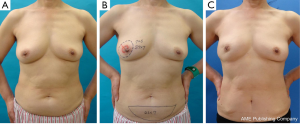
Data were collected until June 2011. Postoperative complications were recorded until the latest time distinguishable by each surgeon (Figures 2-4).
Postoperative outcomes
Complications occurring within 1 month, 1 to 12 months, and over 12 months were recorded and included the following; skin necrosis, delayed wound healing, outflow of fat, and infection.
Patient selection
Two hundred and sixty-two data sheets were collected from 14 institutions for this retrospective study. To investigate postoperative complications, we first excluded 20 patients because of a shorter observation period than 1 month or the lack of complete answers regarding postoperative complications. Two hundred and forty-two and 151 patients were analyzed to determine the incidence of postoperative complications within 1 month and 1 to 12 months, respectively. Before performing statistical analyses for postoperative risk factors, we furthermore excluded 31 and 13 patients from each phase for the following reasons: (I) patients without information on resected breast tissue, and (II) patients without information on implanted FDFG.
Patients, surgery, and oncological variables were analyzed to identify factors associated with postoperative complications for these two study periods: (I) within the first month and (II) 1 to 12 months after surgery. Thus, the numbers of cases used in the statistical analyses were 211 and 124 for the first and second study periods, respectively (Figure 5).
Statistics
A comparison of the distribution of categorical variables between patients with and without complications was performed using the chi-square test. The Mann-Whitney U test was used for continuous variables. In addition, these variables were categorized into two or three groups and analyzed by the chi-square test. Variables with P<0.05 on either the chi-square test or Mann-Whitney U test were applied for further analyses to estimate odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) using a logistic regression model in adjusting for the effect of the experience of the surgeon/plastic surgeon. The risk of postoperative complications with changes in continuous variables was examined by the likelihood ratio test. Significance was defined as P<0.05. All P values are two-sided.
Results
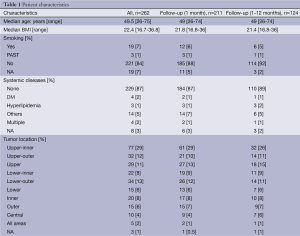
Background and surgical procedure (Table1)
Between October 2003 and December 2010, 262 patients underwent partial/total mastectomy followed by immediate breast reconstruction using FDFG at 14 institutions in Japan. Over that period, 251 patients underwent partial mastectomy and 11 underwent total mastectomy. The indication for this treatment depended on each institution and individual surgeon. The indications for BCS using FDFG were as follows: all patients who were selected to undergo partial mastectomy in 0 institutions; tumor locations in three; for research in one; size and shape of the breast in three; and others in four institutions, respectively.
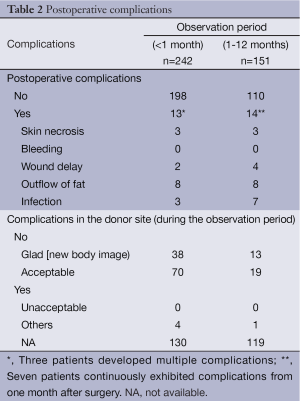
The median age and median body mass index (BMI) of patients were 49.5 (range, 26-75) years and 22.4 (range, 16.7-36.8) years, respectively. The clinical, preoperative, and oncological data of patients are listed in Tables 1 and 2. Twenty-two patients had a history of smoking. Systemic diseases such as diabetes mellitus, hyperlipidemia, and others, were identified in 5, 10, and 14 patients, respectively. Cancer lesions were located in the upper-inner quadrant area in 77 patients, upper-outer in 32, upper (upper-inner and upper-outer) in 29, lower-inner in 22, lower-outer in 34, lower in 15, inner in 20, outer (upper-outer and inner-outer) in 15, central in 10, and all areas in 5, respectively. Preoperative systemic therapy involving chemotherapy and endocrine therapy was administered to 11 and 3 patients, respectively. Incisional biopsy for diagnosis was performed in 4 patients.
Full table
Partial mastectomy was performed in 199 patients, quadrantectomy in 52, and total mastectomy in 11. Sentinel lymph node biopsy and axillary lymph node dissection were performed in 190 and 59 patients, respectively. The fascia of the major pectoralis muscle was completely removed in 109 patients. The thickness of the skin above the resected area was 0-5 mm in 75 patients, 6-10 mm in 20, 11-20 mm in 10, and over 21 mm in 132. The median size of resected breast tissue was 7.8 (range, 3.0-19.5) cm horizontally and 7.0 (range, 3.0-17.5) cm vertically. The median thickness and weight of resected breast tissue were 2.0 (range, 0.3-6.5) cm and 63 (range, 14-230) g, respectively.
In the plastic procedures performed, denuding was achieved with a knife in 247 patients, scissors in 12, and dermatome in 3, respectively. Concerning the preparation of FDFG, denuding was performed ahead of harvesting in 237 patients whereas harvesting was conducted ahead of denuding in 14 patients, respectively. The median size of FDFG was 7.0 (range, 3.0-22.0) cm horizontally and 6.0 (range, 3.0-19.0) cm vertically. The median thickness and weight of FDFG were 2.0 (range, 0.8-8.0) cm and 54.5 (10-215) g, respectively. A closed suction drain or open drain was placed in the implanted area in 210 and 45 patients, respectively.
The median total surgical period and total plastic period were 150 (range, 65-500) minutes and 60 (range, 19-167) minutes, respectively. Median bleeding was 73 (range, 0-630) g.
Chemotherapy was administered as postoperative systemic therapy to 33 patients, endocrine therapy to 135, chemotherapy followed by endocrine therapy to 33, and no treatment in 42, respectively (Table 1). The chemotherapy regime and endocrine therapy were FEC (fluorouracil, epirubicin and cyclophosphamide), CE (epirubicin and cyclophosphamide), Taxane (docetaxel or paclitaxel), TC (docetaxel and cyclophosphamide), AC (doxorubicin and cyclophosphamide), UFT (uracil-tegafur), GEM (gemcitabine), CMF (cyclophosphamide, methotrexate and fluorouracil), Trastuzumab, and AI (aromatase inhibitor: anastrozole or letrozole), TAM (tamoxifen), LH-RH ag (goserelin acetate implant or leuprorelin acetate), tremifen.
Prior to performed analyses, we excluded 20 cases with observation periods within 1 month or incomplete data for postoperative complications (Figure 5).
Postoperative complications (Table 2)
Complications occurred in 13 patients within 1 month of surgery, with skin necrosis in 4, delayed wound healing in 3, outflow of fat in 4, and infections in 2 multiple complications were observed in 3 patients.
Fourteen patients developed postoperative complications between 1 to 12 months after surgery while seven patients continuously exhibited complications from 1 month after surgery as 22 events. Skin necrosis occurred in 3 patients, delayed wound healing in 4, outflow of fat in 8, and infections in 7 patients. Multiple complications were observed in 4 patients.
Fatty melting and wound healing delay were observed in one patient over 12 months after surgery.
All complications were managed conservatively with antibiotics and/or prolonged drainage and/or debridement. Regarding the relationship between follow-up and the overlap of patients with complications, 13 (5.7%) out of 242 patients developed complications within one month of surgery, 7 (4.6%) out of 151 patients between 1 and 12 months after surgery, and one (1.4%) out of 72 patients over 12 months after surgery, respectively. We then examined the relationship between postoperative complications and clinical and technical factors within 1 month and 1-12 months postoperatively. According to the exclusion of insufficient cases (Figure 2), 13 and two patients who developed postoperative complications within 1 month and 1-12 months, respectively, were recruited for further statistical analysis, while the total numbers of patients in each period were 211 and 124, respectively. Two cases were considered to be inappropriate for inclusion in an analysis of the relationship between postoperative complications and clinical factors; therefore, we analyzed 13 out of 211 patients who had postoperative complications between 1 to 12 months after surgery.
Complication rates in 14 institutions
The number of complication-positive cases ranged between 0 and 5 (55.6%). Postoperative complications were not observed in any patients in nine institutions.
Postoperative complications in the donor site (Table 2)
Complications in the donor site were observed in 114 cases only. Thirty-nine patients (14.9%) felt comfortable or good with their new body image, 71 (27.1%) felt that their appearance was acceptable, and 4 (1.5%) felt that it was unacceptable at the donor site.
Univariate cox-regression analysis for postoperative complications
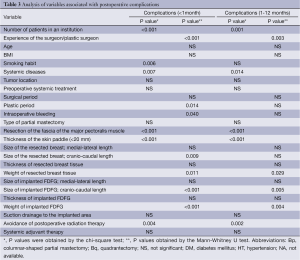
We excluded some cases as insufficient because they lacked detailed surgical data for statistical analyses such as the size and weight of resected breast tissue and implanted FDFG. Two hundred and eleven and 124 cases were examined. The number of surgeries performed at each institution, the experience of the surgeon, smoking habit, systemic diseases, plastic period, intraoperative bleeding, resection of the fascia of the major pectoralis muscle, thickness of the skin paddle, size of resected breast tissue (cranio-caudal length of tissue), weight of resected breast tissue, size of implanted FDFG (cranio-caudal length), weight of implanted FDFG, and avoidance of postoperative radiation for remnant glands were risk factors for within one month (Table 3).
Full table
Multivariate cox-regression analysis for complications within one month of surgery
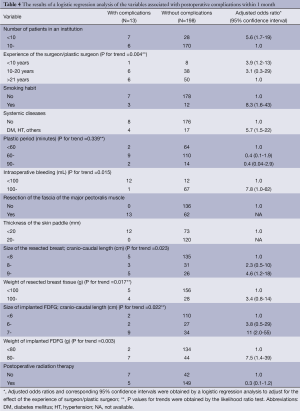
Variables showing a correlation with postoperative complications within one month on either the chi-square test or Mann-Whitney U test were applied to further analyses using a multivariate logistic regression model adjusting for the effect of the experience of the surgeon/plastic surgeon. Further analyses for postoperative complications 1-12 months after surgery were not carried out as there were only two patients with complications during that period. A multivariate analysis revealed that the weight of resected breast tissue, size of implanted FDFG (cranio-caudal length), and weight of implanted FDFG were associated with a higher likelihood of postoperative complications after OBS combining partial mastectomy with immediate volume replacement using FDFG (Table 4).
Full table
Discussion
BCT has rapidly become the first-line procedure for early-stage breast cancer, ensures local control, and produces acceptable cosmetic results (1,2). Cosmetic results have been associated with psychological morbidity in patients who have undergone BC (2-4). OBS, which combines the concepts of both oncologic and plastic surgeries, is becoming more common, especially in Western countries (15,16). Many different techniques are performed in OBS, one of which involves the careful planning of skin and parenchymal excisions, reshaping of the gland after parenchymal excision, and repositioning of the nipple areola complex to the center of the breast mound with or without a correction to the contralateral breast for better symmetry (17). According to Hoffmann’s classification of OBS, there are four categories in BCT due to technical complexity and difficulty. They recommended that an oncoplastic procedure should be performed for cosmetic reasons if the breast defect after partial mastectomy was over 25% of the total size of the breast (18).
Autologous FDFG has been used sporadically for soft tissue augmentation. Previous studies reported that an ideal reconstructive technique in the field of the surgical treatment of head and neck diseases would be easy, inexpensive, single-stage, and autologous (19,20). BCS and immediate reconstruction using FDFG were shown to be effective for selected patients with small breasts and a slim body in a retrospective study at a single institution (13,21). A study has not yet been conducted to compare other breast reconstruction methods using vascularized flaps and fat injections with this method (14). In a previous study, the operative procedure and cosmetic results were retrospectively compared among three groups according to the reconstructive procedure that was used for the defect following partial mastectomy. Patients receiving immediate volume replacement using a mini flap of the latissimus dorsi (LD group) achieved better cosmetic results than those receiving only rotation and fixation of the parenchymal adipose tissue or gland to repair the defect. Disadvantages observed in the LD group over the FDFG group were longer operation durations, more bleeding, higher rates of postoperative complications, and longer hospital stays. Fat injections represent a complementary technique that is ideal for autologous reconstruction using the LD flap because its muscle and fat act as an ideal recipient site for fatty tissue grafts (22). It is an excellent technique that can be applied to all patients, except those with no potential fat deposits. Fat injections can also be used for implant reconstruction, replacement of an implant, and revision after a vascularized flap. Lipofilling and adipose tissue containing flap (e.g., TRAM, DIEP) have been well known as vascular-rich and oncologically safe grafts to repair a defect after partial and total mastectomy. Unfortunately there is no literature in which the clinical and basic results were compared between those techniques and the immediate volume replacement using FDFG. To compare the results and discuss the differences between them, the further research should be needed. Only one literature used a rat model implanted FDFG reported the histological findings of implanted FDFG, the vascularity and apoptotic resistance (23). From their conclusion, we presume that the implanted FDFG in the clinical case is maintained by the vascularization of a certain degree.
Immediate volume repair for the partial defect after BCS in each institution was as follows; OBS using FDFG was selected for all patients who were indicated for BCS in 0 institution; the other volume replacement technique using autologous tissue such as a latissimus dorsi muscle flap or local tissue flap was selected in two institutions; volume displacement using parenchymal breast tissue was selected in nine institutions; no volume replacement or displacement was selected in two institutions.
The relationship between postoperative complications and clinical and technical factors after surgery was assessed using a univariate cox-regression analysis and the results obtained identified the number of patients in each institution, the experience of the surgeon/plastic surgeon, BMI, smoking habit, resection of the fascia of the major pectoralis muscle, thinner (<20 mm) skin envelope, larger FDFG in the cranio-caudal length (>6 cm), and devices used for denuding as significant risk factors for postoperative complications. A multivariate cox-regression analysis revealed that the experience of the surgeon/plastic surgeon, heavier breast tissue (>100 g), larger FDFG in the cranio-caudal length (6 cm), and thicker FDFG (>3 cm) were significant risk factors for postoperative complications. No significant differences were observed in postoperative complications or oncological factors such as the tumor size, location, and preoperative and postoperative therapy for cancer control. The number of complication-positive cases ranged between 0 and 5 (55.6%) in 14 institutions. No postoperative complications were observed in any patients in 9 institutions, whereas the incidence of these complications was high in one institution (55.6%). Although a learning curve or skill in avoiding postoperative complications may exist, no significant difference was noted between postoperative complications and the experience of the breast surgeon or plastic surgeon by a multivariate cox-regression analysis; however, since this study was retrospective, not prospective, bias may exist that should be taken into consideration. Over 50% of all tumors were located in the upper-inner, upper-outer, or upper area. Although partial mastectomy with immediate breast reshaping using FDFG provided excellent results for patients with a slim body and diagnosed early with breast cancer in the upper-inner quadrant area (13), it currently remains unknown whether partial mastectomy can be safely performed in this area in a patient with slim body and small breasts. The results to avoid postoperative complication in this study maybe introduce rather than contra-indication on such cases.
In the upper areas (A or C), we trimmed the thickness of FDFG so that it easily fit the defect. FDFG was made thinner to replace the upper portion of the breast, and thicker to replace the area under the nipple-areolar complex. In the lower areas (B or D), we could not repair the defect to adjust the thickness of FDFG and resected breast tissue because of a limitation in the thickness of the donor site of FDFG. This procedure was originally performed on and indicated for slim patients with early breast cancer in the upper areas of small breasts (13). However, this study revealed that this procedure could be performed on patients with lower lesions (72 cases) and for patients with a BMI over 25 (46 cases). Furthermore, these were not risk factors for postoperative complications.
The BMI of our patients ranged between 16.7 and 36.8 with an average of 22.4. Therefore, we cannot currently confirm that OBS is adequate for Western women with higher BMI. We can only state that even patients with high BMI were able to undergo BCS with immediate volume replacement using FDFG if their resected breasts had a cranio-caudal length of under 8 cm, a weight under 100 g, implanted FDFG had a cranio-caudal length of under 6 cm, the weight of implanted FDFG was under 80 g, and postoperative radiation therapy was administered. Under these conditions, early breast cancer without widely spreading intraductal components that is located in the upper-inner quadrant area in which breast thickness is relatively thin in any patient with high or low BMI may be good indications in Western women.
In this study, the percentage of irradiation at each institution ranged between 0% (two institutions that enrolled four and five patients to this study, respectively) and 100%. A reverse correlation was observed between postoperative radiation therapy and postoperative complications. Although the rate of complications was predicted to be high in the irradiation group, the reverse was observed. The prolonged development of postoperative complications may account for why postoperative radiation therapy was not administered adequately. To resolve this question, further studies should be planned prospectively with particular indications for surgical, systemic, and radiological treatments. Adverse results to control tumor progression should also be analyzed individually in the next step of this study.
A disadvantage of this procedure is the horizontal scar on the lower abdomen (13). Although 161 patients left the answer box blank, 39 answered that they were happy with the results, 71 said they were acceptable, and 4 were not happy. Middle-aged to elderly Japanese women are not in the habit of wearing bikinis, and sunbathing and swimming in the sea are not popular activities. Therefore, this may explain the low complication rate regarding the donor site.
This study had important limitations. It was a retrospective study and data was collected from several institutions retrospectively. A prospective study needs to be conducted in order to demonstrate that OBS combining partial mastectomy with immediate volume replacement using autologous FDFG is a feasible procedure for selected cases. In addition, there was a lack of cosmetic evaluations. Although one institution reported cosmetic advantages over other techniques for selected patients, we did not examine this point. Further prospective or randomized and larger analyses are needed regarding cosmetic results. In the present study, data were collected without any pilot study for the protocol used or training for this procedure; therefore, the quality of the technique and skill set were completely dependent on the surgeon. This is the first study to describe this procedure in multiple institutions.
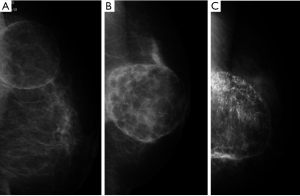
Fat necrosis can typically develop within 2-3 years of surgery or longer. Therefore, the relationship between the extent of fat necrosis and worse cosmetic results needs to be determined over a longer observation period. Implanted FDFG had three patterns on mammography; a mass with lower density than fatty tissue; a mass with the same density as parenchymal tissue; and a mass with coarse calcifications (Figure 6). After analyzing the questionnaire of this study, we were unable to detect any difference in the hardness of FDFG between the radiated and irradiated groups. Appropriate guidelines should be established for this technique in order to reduce postoperative complications. A longer follow-up period of 5 years is also essential for assessing the delayed development of complications and cosmetic results. A previous study conducted in one institution, showed that postoperative cosmetic problems and fibrous degeneration of FDFG are associated with this procedure (13,21). In the present study, postoperative breast form was excluded from the postoperative complications examined. We should have clarified postoperative complications based on the experience of the surgeon, techniques used, procedures performed, and treatment-related factors by collecting data from non-selected institutions. We are aware of the necessity of a further study to objectively evaluate the hardness of the breast and cosmetic results. In the present study, we could not differentiate fat necrosis from degenerated FDFG using ultrasonography or other objective findings. Image evaluations by central judgments and histopathological analysis of implanted FDFG are also required. The experience of the included institutions with this procedure varied widely, and may have impacted on the results obtained. Future studies should involve a larger sample size and longer follow-up, and also examined the various clinical applications of our technique.
Conclusions
OBS combining partial mastectomy and immediate volume replacement using FDFG can be performed safely with a low incidence of postoperative complications; however, the complete avoidance of postoperative complications is essential. A learning curve under an experienced surgeon may be necessary for young breast surgeons or plastic surgeons. Immediate breast volume replacement using a FDFG after breast cancer surgery should be done for selected patients, and also the prospective and larger investigations are warranted for the establishment of appropriate guidelines.
Acknowledgments
The authors thank Dr. Shoshu Mitsuyama, President of Kitakyushu City Hospital, Prof. Shigemi Sakai, Department of Plastic Surgery, Mita Hospital, Prof. Shoji Natsugoe and Dr. Heiji Yoshinaka, Department of Digestive Surgery, Breast and Thyroid Surgery, Kagoshima University, Dr. Teiji Umemura, Kiwa Hospital, Dr. Hiroshi Kaise, Tokyo Medical University, and Dr. Hiroshi Yagata, St. Luke’s Hospital, for their advice on our study.
Disclosure: The authors declare no conflict of interest.
References
- Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995;333:1456-61. [PubMed]
- Rowland JH, Desmond KA, Meyerowitz BE, et al. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. J Natl Cancer Inst 2000;92:1422-9. [PubMed]
- Fujishiro S, Mitsumori M, Kokubo M, et al. Cosmetic results and complications after breast conserving therapy for early breast cancer. Breast Cancer 2000;7:57-63. [PubMed]
- Al-Ghazal SK, Blamey RW, Stewart J, et al. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol 1999;25:566-70. [PubMed]
- Pezner RD, Patterson MP, Hill LR, et al. Breast retraction assessment. Multiple variable analysis of factors responsible for cosmetic retraction in patients treated conservatively for stage I or II breast carcinoma. Acta Radiol Oncol 1985;24:327-30. [PubMed]
- Van Limbergen E, Rijnders A, van der Schueren E, et al. Cosmetic evaluation of breast conserving treatment for mammary cancer. 2. A quantitative analysis of the influence of radiation dose, fractionation schedules and surgical treatment techniques on cosmetic results. Radiother Oncol 1989;16:253-67. [PubMed]
- Sacchini V, Luini A, Tana S, et al. Quantitative and qualitative cosmetic evaluation after conservative treatment for breast cancer. Eur J Cancer 1991;27:1395-400. [PubMed]
- Vrieling C, Collette L, Fourquet A, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC'boost vs. no boost' trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. Radiother Oncol 2000;55:219-32. [PubMed]
- Tanaka S, Nohara T, Nakatani S, et al. Esthetic result of rhomboid flap repair after breast-conserving surgery for lower quadrant breast cancer lesion with skin invasion: report of two cases. Surg Today 2011;41:832-6. [PubMed]
- Ohuchi N, Harada Y, Ishida T, et al. Breast-Coserving Surgery for Primary Breast Cancer: Immediate Volume Replacement Using Lateral Tissue Flap. Breast Cancer 1997;4:135-141. [PubMed]
- Kijima Y, Yoshinaka H, Owaki T, et al. Immediate reconstruction using inframammary adipofascial flap of the anterior rectus sheath after partial mastectomy. Am J Surg 2007;193:789-91. [PubMed]
- Kijima Y, Yoshinaka H, Funasako Y, et al. Immediate reconstruction using thoracodorsal adipofascial flap after partial mastectomy. Breast 2009;18:126-9. [PubMed]
- Kijima Y, Yoshinaka H, Owaki T, et al. Early experience of immediate reconstruction using autologous free dermal fat graft after breast conservational surgery. J Plast Reconstr Aesthet Surg 2007;60:495-502. [PubMed]
- Kijima Y, Yoshinaka H, Funasako Y, et al. Immediate breast reconstruction using autologous free dermal fat grafts provides better cosmetic results for patients with upper inner cancerous lesions. Surg Today 2011;41:477-89. [PubMed]
- Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction (TSIR) in breast cancer patients. Perspect Plast Surg 1998;11:71-106.
- Audretsch WP, Rezai M, Kolotas C, et al. Onco-plastic surgery: “target” volume reduction (BCT-mastopexy), lumpectomy reconstruction (BCT-reconstruction) and flap-supported operability in breast cancer. In: Proceedings of the 2nd European Congress on Senology; October 1994; Vienna, Austria; Moncuzzi, Bologna, Italy, 139-57.
- Masetti R, Pirulli PG, Magno S, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer. Breast Cancer 2000;7:276-80. [PubMed]
- Hoffmann J, Wallwiener D. Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer 2009;9:108. [PubMed]
- Davis RE, Guida RA, Cook TA. Autologous free dermal fat graft. Reconstruction of facial contour defects. Arch Otolaryngol Head Neck Surg 1995;121:95-100. [PubMed]
- Nosan DK, Ochi JW, Davidson TM. Preservation of facial contour during parotidectomy. Otolaryngol Head Neck Surg 1991;104:293-8. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Clinical and pathologic evaluation of implanted free dermal fat grafts after breast cancer surgery: a retrospective analysis. Surgery 2012;151:444-55. [PubMed]
- Delay E. Lipomodelling of the reconstructed breast. Chapter 66, Surgery of the breast – Principles and Art. Ed Spear S.L. Second Edition 930-946 Lippincott Williams & Wilkins, Philadelphia 2006.
- Mizoguchi T, Kijima Y, Hirata M, et al. Histological findings of an autologous dermal fat graft implanted onto the pectoralis major muscle of a rat model. Breast Cancer 2014. [Epub ahead of print]. [PubMed]



