Achieving ideal breast aesthetics with autologous reconstruction
Introduction
Modern viewpoints of breast reconstruction following mastectomy have emphasized quality, outcome, and patient satisfaction as the ultimate endpoints. This is true for both prosthetic and autologous reconstruction. Traditional concepts of breast reconstruction had focused on creation of a mound that may or may not have resembled the natural breast contours. Modern concepts of breast reconstruction however focus on the creation of a breast that resembles if not improves upon the natural breast shape. Breast reconstruction has evolved from a purely reconstructive endeavor to one that places importance on artistry and aesthetics. This is especially true for autologous reconstruction because there is no template or prosthetic device to shape the breast mound. Autologous reconstruction requires a skill set that emphasizes shaping, configuring, and positioning the new breast based on patient expectations, body habitus, and surgeons’ skill.
The evolution of autologous reconstruction has placed in increasing emphasis on reducing donor site morbidity and improving breast aesthetics. Autologous options have expanded and now include the traditional pedicle flaps as well as the newer microvascular perforator flaps. Pedicle options such as transverse rectus abdominis musculocutaneous (TRAM) and the latissimus dorsi (LD) flaps continue to remain viable options and are capable of producing excellent aesthetic outcomes. Perforator flaps such as the deep inferior epigastric perforator (DIEP), superior gluteal artery perforator (SGAP), inferior gluteal artery perforator (IGAP), transverse upper gracillis (TUG), and the profunda artery perforator (PAP) flap are also equally capable of producing excellent aesthetic outcomes. This manuscript will focus on many of these autologous options and provide a template or framework based on the authors experience on achieving ideal breast aesthetics following in the setting of total breast reconstruction following mastectomy.
It is important to have an appreciation of ideal breast aesthetics when embarking on reconstruction. The position of the breast on the chest wall should serve as footprint for the breast to be reconstructed. The natural shape or cone of the breast should be appreciated in order to estimate the amount of skin that will be required to achieve ideal proportions. It is important to understand the relationship of the torso to the breast in order to reconstruct a breast that will be appropriate for the patient’s frame and body habitus. The ultimate goal is to create symmetry, proportion, and contour.
Traditional teaching for breast shaping occurs during residency or fellowship in plastic and reconstructive surgery. It is based on an apprenticeship or mentorship model where resident or fellow sees, does, and teaches. Initially the technical aspects of the operation are the most important and require mastery; however, with surgical maturation, the aesthetic aspects become as important and thus the artistic nature of breast reconstruction comes to fruition. The three-step principalization of breast reconstruction was described by Blondeel et al. and is an excellent foundation from which to start ones journey to achieving ideal breast aesthetics (1-4).
The concept is based on the breast footprint, the conus, and the skin envelope. The footprint is unique for each woman and defined and fixed for each breast. The borders of the footprint include the clavicle, lateral edge of the sternum, anterior axillary line, and the inframammary fold. It is important to appreciate that the footprint is stable and does not change with weight gain or loss. The footprint represents the foundation for the conus. The conus represents the 3-dimensional shape, volume, projection, and contour of the breast. This will vary with weight gain or loss. The conus is typically characterized with a lower pole prominence. In general, the ideal breast proportions based on the ratio of lower and upper pole is defined as 55% from the nipple to the IMF and 45% from the nipple to the upper edge of the breast. The final component is the skin envelope. In the setting of immediate reconstruction, the quality and quantity of skin is important. Skin quality is affected by previous surgery, radiation, scar, and vascularity.
Preoperative considerations
Preoperative factors are important considerations prior to autologous breast reconstruction (5). Patient co-morbidities must be assessed and managed. Conditions such as diabetes mellitus, hypertension, and cardiac disease must be optimized. Tobacco use is strictly discouraged. Preoperative or postoperative radiation is also noted and reviewed. Physical examination must include the breast, donor site, presence of scars, quality of tissue, as well as relevant measurements such as the base width and location of the nipple areolar complex. The footprint of the breast must be appreciated, as this will be the template for the new breast mound.
Timing of autologous reconstruction
Breast reconstruction with autologous tissue can be performed immediately following mastectomy, on a delayed basis following mastectomy, or following reconstruction using prosthetic devices. It can also occur prior to radiation therapy or after radiation therapy. There are several strategies that are useful for reconstructive surgeons in order to optimize aesthetic and surgical outcomes.
In cases of immediate breast reconstruction, the quality of the mastectomy will have a significant impact on the quality of the reconstruction. Most mastectomies are performed using either skin sparing techniques or nipple areolar sparing techniques. With both approaches, the vascularity of the remaining mastectomy skin flaps must be maintained. Incisional approaches for the mastectomy may be apical, inframammary, or lateral areolar. When there has been extensive undermining of the mastectomy flaps, the natural borders are reestablished by suturing the inframammary and lateral mammary folds back to the chest wall. If the vascularity of the mastectomy skin flaps is compromised, the edges are excised until normal bleeding is observed. With all of these approaches, autologous reconstruction can be performed with excellent aesthetic outcomes.
An important and sometimes overlooked aspect of achieving ideal breast aesthetics is to make the necessary adjustments to the skin envelope following mastectomy in the setting of immediate reconstruction. When the mastectomy skin is in excess, it should be debrided to fit the flap. In the setting of a skin sparing mastectomy, the circular excision pattern can be further excised and adjusted to fit the desired skin paddle of the flap with a purse string suture technique. An alternative option is to partially inset the flap medially and then excise the lateral mastectomy skin such that a laterally based “lollipop” pattern is created. With nipple sparing mastectomy it is important to ensure that enough donor tissue is present to fill the skin envelope.
In women considering delayed reconstruction, there are several factors that must be considered. In patients that have had radiation therapy prior to autologous reconstruction, adequate time must elapse in order to allow the tissues and vascularity to recover (6). This is typically 6-12 months. In those patients that will receive radiation therapy following reconstruction, consideration must be given to the type of reconstruction being performed. It is known that one of the long-term effects of radiation on breast tissue and fat is shrinkage and distortion. In order to eliminate these effects, many reconstructive surgeons have adopted the delayed-immediate approach as a means to avoid radiation damage to the flap (7). A subpectoral tissue expander is placed immediately following the mastectomy and becomes the conduit for the radiation beams. Following radiation, the device is removed and replaced by a flap. Most tissue expanders and implants are placed totally or partially behind the pectoralis major muscle. When these devices are removed, the pectoralis major muscle is always placed back on the chest wall such that the flap is positioned on top of the muscle. This will allow for optimal aesthetics. In addition, radiated tissue that has been extensively damaged or fibrotic is usually excised. In these cases, the inferior edge of the flap usually becomes the upper aspect of the inframammary fold.
Breast shaping with abdominal flaps
Abdominal flaps such as the TRAM, DIEP, and SIEA flaps remain the most commonly used flaps for autologous reconstruction (8,9). The reasons for this are that the abdominal donor site often provides ample tissue for a unilateral or bilateral reconstruction, is convenient for flap harvest, allows for reconstruction as a pedicle flap or free tissue transfer, has dimensions that can be tailored to the mastectomy specimen, can be used for immediate or delayed reconstruction, and has superior pliability that facilitates optimal breast shaping. The vascularity of abdominal flaps is based on the superior epigastric (pedicle TRAM), inferior epigastric (free TRAM, DIEP), and superficial epigastric (SIEA) systems. As with all flaps, perfusion must be assessed using conventional methods such as capillary refill, arterial bleeding from the distal edge, and surface temperature. Advanced technologies are also used to assess perfusion such as fluorescent angiography and near infrared spectroscopy. The zones of perfusion are important and are subdivided into four zones. The perfusion is optimal when the skin and fat are in close proximity to the source vessel; thus zone 1 is the best perfused and zone 4 is least perfused.
Shaping the free abdominal flap
There are several shaping advantages using free flaps for breast reconstruction. The flap is not tethered to the donor site muscle that allows for optimal positioning on the chest wall. The recipient vessels are usually the internal mammary and also the thoracodorsal. The choice of vessel is surgeon dependent. The internal mammary vessels are usually exposed at the level of the 3rd or 4th costal rib segment and are the preferred recipient vessels for the majority of microsurgeons. The thoracodorsal vessels are exposed proximal or distal to the serratus branch. Because these vessels are laterally based, bilateral reconstructions may sometimes result in a slight medial/sternal volume deficiency. This is usually not a problem with unilateral cases because zone 3 can supplement the medial breast. With either recipient vessel couples to the flap vessel, there is usually ample length of the vascular pedicle such that the flap can be shaped and contoured without impediments.
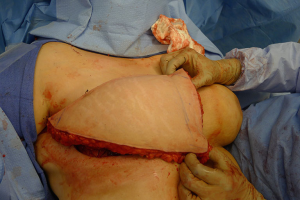
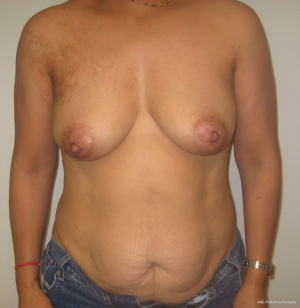
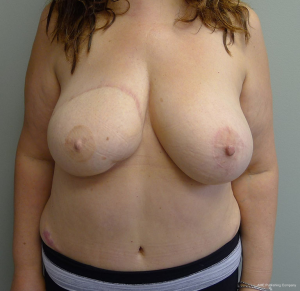
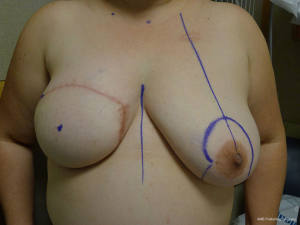
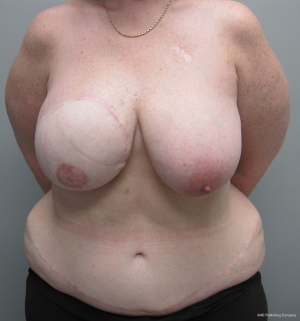
With bilateral reconstruction, the abdominal donor tissue is bisected at the midline such that each flap contains a zone 1 and 2. With bilateral reconstruction, the weight of the mastectomy specimens is usually not important because that volume of flap is fixed. The medial edge of the flap is usually positioned along the sternal border and the lateral aspect of the flap is positioned on the lateral chest wall (Figure 1). Suturing of the flap to the chest wall is sometimes necessary with immediate reconstruction especially when the footprint of the old breast is larger than the footprint of the flap. These sutures can be placed superomedially, inferomedially, and laterally. With delayed reconstruction, the dimensions of the created subcutaneous pocket are made to match that of the flap. Figures 2 and 3 illustrate a patient following bilateral skin sparing mastectomy and immediate reconstruction with bilateral DIEP flap.
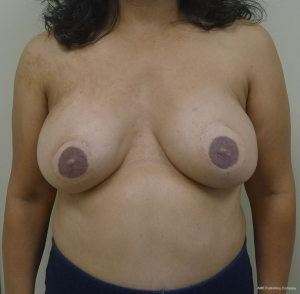
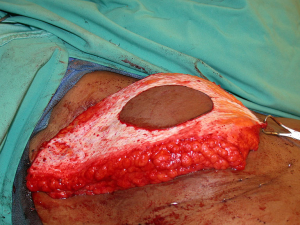
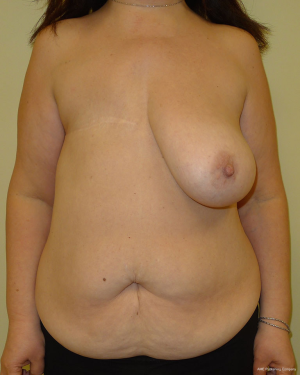
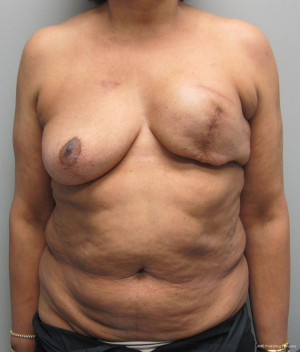
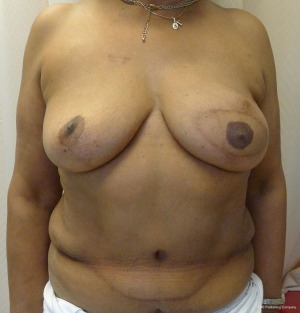
With a unilateral reconstruction, it is important to achieve symmetry with the opposite breast. Assessment of patient expectations is critical because it is important to know if the opposite breast will be reduced, augmented, or left as is. The opposite breast is used as a template for the reconstruction. Typically with a unilateral reconstruction, zones 1-3 and sometimes zone 4 are utilized depending on the amount of tissue required. Because there is usually more tissue with a unilateral flap (zones 1-3) compared to the bilateral flap (zones 1-2), there are more shaping options. The flap can be folded in a conical fashion or it can be folded laterally such that apical portion (zone 2) of the flap is tucked under zone 1 with zone 3 of the flap being positioned along the sternal border. With both maneuvers, the goal is to provide better projection. Suturing the flap laterally is always necessary and suturing the flap along the medial border is sometimes necessary. Figures 4 and 5 illustrate a patient following delayed reconstruction with unilateral DIEP flap.
Shaping the pedicled abdominal flap
Shaping of the pedicle TRAM flap is slightly different than that of the free flap. This is because the adipocutaneous component of the flap is attached to the rectus abdominis muscle. With a unilateral TRAM flap the flap is based on either the ipsilateral or contralateral rectus abdominis muscle. With a bilateral pedicle TRAM flap, the flaps are based on the ipsilateral rectus abdominis muscle. The advantage of a flap that is tethered to the abdomen and chest is that it is less likely to migrate when inset; therefore, extensive suturing to the chest wall is less likely. Like the free flaps, the pedicle flap can include zones 1-3; however, it is important to appreciate that the vascularity to the distal aspects of the flap may not be as robust compared to the free flaps based on the perfusion dynamics of the primary source vessels. In some patients with a thick adipose layer, the sub-Scarpa fat is sometimes excised to minimize the likelihood of fat necrosis. The orientation of the flap on the chest wall is typically with the cut edge of zone 2 or 3 being placed along the sternal border.
Flap insetting
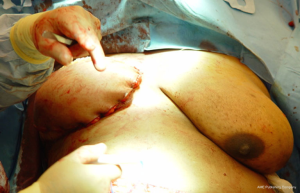
When insetting the flap following a unilateral or bilateral reconstruction, it is recommended to sit the patient upright to approximately 45 degrees to assess the position, symmetry, contour, and projection of the breast. In cases of a skin-sparing mastectomy, the skin territory to be exteriorized is delineated and the remainder of the flap is de-epithelized (Figure 6). When a nipple-sparing mastectomy has been performed, a doppler is used to identify an arteriovenous signal and delineated with a 2 cm circle. The remainder of the flap is de-epithelized and the skin paddle is exteriorized. With free flaps, the vascular pedicle must be inspected to ensure that it is not twisted or kinked. With pedicle flaps, the tunneling of the flap can sometimes compress the muscle and blood supply; therefore, it is imperative to reassess the perfusion of the flap to ensure that the perfusion is intact. With delayed reconstruction, the lower mastectomy skin is usually excised and the inferior edge of the autologous flap is used to recreate the inframammary fold (Figure 7). Figures 8 and 9 illustrate a patient following delayed unilateral breast reconstruction with a muscle sparing free TRAM flap.
With both skin-sparing and nipple-sparing mastectomy, the flap can be monitored using traditional monitoring techniques that include hand held doppler, assessment of capillary perfusion, and skin turgor. Skin closure is always performed in a layered fashion using absorbable dermal and subcuticular sutures.
Breast shaping with latissimus dorsi (LD) flaps
The LD flaps can be used for immediate or delayed reconstruction as well as for unilateral or bilateral reconstruction (10-12). This flap has been is considered by many plastic surgeons to be a workhorse flap in that it is useful in a variety of situations and can provide predictable outcomes. Although traditionally performed as a pedicle musculocutaneous flap, there are new variations based on the perforator concept that allow transfer of the adipocutaneous component of the flap on a single vascular pedicle without sacrificing the LD muscle. This is known as the thoracodorsal artery perforator flap or TDAP. Because of the limited quantity of fat harvested with this flap, it is commonly combined with an implant for volume and shape. These devices can be placed immediately at time of flap transfer or on a delayed basis as necessary.
Immediate reconstruction with the LD flap
As with all reconstructions, the volume and skin requirements of the new breast are assessed and juxtaposed in relation to the estimated flap volume. Because the LD flaps provides a limited quantity of skin and fat, the use of a prosthetic device is sometimes required. In making this decision, it is important to assess the footprint, conus, and skin envelope on the natural breast. In the case of a volume deficiency, a tissue expander or permanent implant can be considered. This will also help to augment the projection of the breast in order to better define the desired conus. Other methods to obtain additional volume using the LD flap include beveling the fat away from the skin paddle in order to increase the quantity of fat harvested from the back. This is known as an extended LD flap (11). In the case of a skin deficiency following mastectomy, the skin territory can be replaced using the back. This skin paddle is usually oriented horizontally or obliquely along the resting skin tension lines.
There are several decision points when considering use of the LD flap for breast reconstruction. The decision regarding denervation of the LD muscle is controversial. Some surgeons prefer to leave the thoracodorsal nerve intact to prevent muscle atrophy and to maintain greater volume. Others however, prefer to divide the nerve in order to prevent any animation that may occur with a contracting muscle. The LD muscle can be harvested in total or in part based on the volume requirements. In cases of total breast reconstruction, the entire muscle can be harvested; however, in cases of partial breast reconstruction only a small segment of the muscle may be removed. The harvested LD flap is then passed through a high tunnel from the posterior thorax to the anterior chest wall corresponding to the desired footprint. Patient positioning is also an important consideration. With a unilateral reconstruction, the patient is placed in the lateral decubitus position such that the flap harvest and preparation of the recipient site can occur simultaneously. With a bilateral reconstruction, the flaps can be harvested simultaneously with the patient in the prone position (10). Insetting the flap requires that the patient be turned to the supine position.
Shaping the LD flap is sometimes achieved using the flap alone but more often than not requires the use of a prosthetic device. The device can be placed in the partial subpectoral or prepectoral space. When subpectoral, the edge of the LD can be sutured to the inferior edge of the pectoralis major muscle. This will provide for a larger pocket to accommodate a larger device. When prepectoral, the device is usually covered with the LD muscle itself. The devices can be either a tissue expander or a permanent implant depending on the reconstructive requirements. The advantages of a tissue expander are that the surrounding soft tissues can be expanded to provide a larger reconstruction. In addition, further shaping and contouring can be achieved at the second stage when the tissue expander is exchanged for a permanent implant. The disadvantage of a tissue expander is that in some cases, the thickness of the LD flap can complicate localization of the expansion port. A remote access port rather than an integrated port is an alternative to mitigate this situation.
Delayed reconstruction with the LD flaps
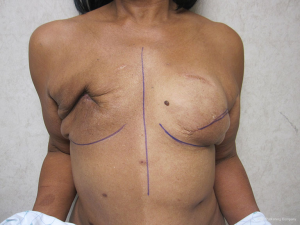
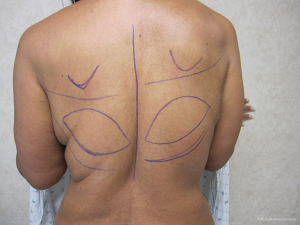
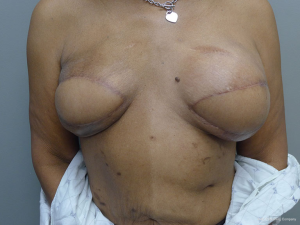
Delayed reconstruction with the LD flap can be performed using the 1- or 2-stage techniques as described above. However, it is this author’ preference to use a 3-stage technique. The first stage involves transfer of the LD muscle to recreate and establish the footprint of the breast, provide a small conus, and to increase the available skin envelope. The second stage involves placement of a tissue expander in the pre or subpectoral space to expand the conus and skin envelope. The third stage involves removal of the expander and placement of a permanent implant. The soft tissue envelope is contoured and shaped to recreate a more natural breast mound. This 3-stage approach is generally recommended for women that have had either failure of an abdominal flap, premature removal of a prosthetic device due to infection or in women that have had previous radiation therapy. The staged approach allows for improvement of the recipient site by the transfer of vascularized muscle and fat. The use of devices can then be used to expand and contour the new breast mound. Figures 10-12 illustrate a patient that had delayed bilateral LD musculocutaneous flaps utilizing the 3-stage approach.
Breast shaping with gluteal or thigh flaps
The final category of breast shaping will include the gluteal and thigh based flaps. These will be grouped together because these flaps are usually less voluminous than the abdominal counterparts and immediate shaping is not always possible. Because these flaps are remote from the breast, they can only be used as a free tissue transfer. Although effective, most surgeons consider these flaps to be a second choice in the event that the abdomen is not a suitable donor site either because it has been previous used, the patient is too thin, or prior operations/scars preclude its use.
Gluteal flaps
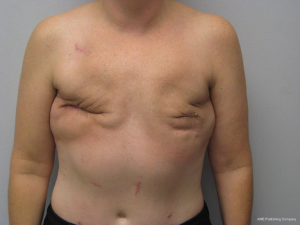
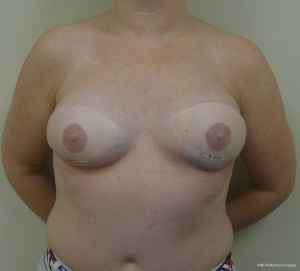
Gluteal flaps include the perforator (SGAP and the IGAP) as well as the musculocutaneous (gluteal) flaps (13-15). Both the upper and lower buttock regions can be used and provide similar quantities of skin and fat. These flaps tend to be thicker than most LD and abdominal flaps in women with BMI <30 and are usually not considered in women with a BMI of >35. These flaps tend to have a shorter vascular pedicle than the abdominal flaps and optimal positioning on the chest wall may be compromised. The dimensions of these flaps are usually based on the gluteal dimensions and typically range from 12 to 25 cm in length and 6 to 10 cm in width. Gluteal thickness in patients that are candidates for these flaps tends to range from 4 to 8 cm. For this reasons, many of these flaps cannot be coned or shaped and are therefore inset as it. Secondary contouring of these flaps is usually necessary. However, in some cases, extended or re-designed gluteal flaps can be harvested that will allow for coning or better shaping of these flaps (15). In cases of delayed reconstruction where a gluteal flaps is considered, pre-expansion of the breast skin to provide an increased skin envelope can improve aesthetic outcome (14). Figures 13 and 14 illustrate a woman preoperatively and following staged bilateral SGAP flaps.
Thigh flaps
Flaps derived from the medial and posterior thigh are frequently considered when the abdomen is not a suitable donor site (16-18). Medial thigh flaps include the transverse musculocutaneous gracillis flap and posterior thigh flaps include the PAP flaps. The medial thigh flaps tend to be longer in width and shorter in height compared to abdominal and latissimus flaps and usually less bulky than gluteal flaps. Typical dimensions are 25-30 cm in length and 8-10 cm in height. Medial thigh flaps typically able to reconstruct a breast of mild to moderate volume (16,18). The dimensions of the flap can make inset challenging and ultimately necessitate a greater revision rate both for the ipsilateral and contralateral breast. The use of this flap is highly advocated for bilateral reconstructions when symmetry can be more easily achieved. Most medial thigh flaps will include a segment of the gracillis muscle whereas the posterior thigh flaps do not include any muscle.
Shaping the breast using medial thigh flaps has been described (17). The segment of gracillis muscle is usually placed along the area where the cartilaginous rib harvest has occurred to minimize visibility of this. Because of the added length of these flaps, they lend themselves nicely to coning by suturing the anterior and posterior edge of the flap together. The coned flap is positioned on the chest wall to ensure adequate coverage of the footprint and to generate adequate projection.
The posterior thigh also known as the PAP flap is rapidly becoming one of the more popular alternatives to the abdomen (19). Like the medial thigh flap it tends to be long and thin with dimensions that range from 20 to 30 cm by 6 to 10 cm. The vascular pedicle is 7-13 cm in length that permits for optimal placement of the flap on the chest wall as well as use of the internal mammary or thoracodorsal recipient vessels. The flap, by nature of its dimensions and elliptical design, can be easily shaped into a cone to provide optimal projection. These tissues are more pliable than the gluteal tissue and sometimes more pliable than the abdominal tissue that facilitates coning and achieving ideal aesthetics. Flap weight typically ranges from 250 to 600 grams and can be used unilaterally and bilaterally. The incidence of fat necrosis has been generally <10%. Secondary recontouring is usually not necessary.
Secondary revisions
Secondary revisions are often necessary following autologous reconstruction (20). This may be to restore volume, contour, and position. This may include both breasts in the setting of a bilateral reconstruction as well as the ipsilateral and contralateral breast in the setting of a unilateral reconstruction. Various techniques are available for the reconstructed breast that includes soft tissue recontouring, fat grafting, burying the flap, and implant placement. Achieving symmetry with a non-reconstructed breast can be achieved by augmentation, mastopexy, and reduction mammaplasty.
The reconstructed breast
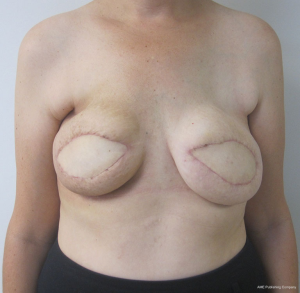
Perhaps the most common method of revision is to recontour the soft tissue by direct excision of skin and fat as well as tissue rearrangement (20). This can be performed to reduce the volume, improve the shape, reposition the breast on the chest wall, and to better define the inframammary or lateral mammary folds. The use of autologous fat grafting to correct contour deformities and to improve skin quality has become another common method of revision that has achieved success. The placement of fat along the upper pole of the breast and chest wall is an ideal method to achieve natural contours. Fat grafting has also been used in radiated breasts where skin damage is present. The ability of fat and stem cells to regenerate, hydrate, and revascularize damaged skin has been described (21). For the autologous reconstruction that is too ptotic, the technique of burying the flap has demonstrated success. The skin territory is outlined with an elliptical extension and de-epithelized. The mastectomy skin flaps are undermined superiorly and inferiorly and then re-approximated. Figures 15 and 16 illustrate a patient that has had both flaps de-epithelized and buried under the superior and inferior mastectomy skin flaps.

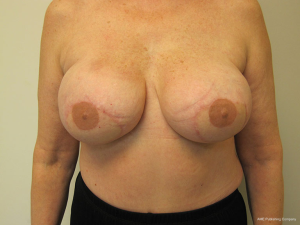
For the reconstructed breast that is deficient in volume, two methods of correction are commonly employed. The first is to fat graft the substance of the flap and the second is to place a small implant under the flap. The implant is usually saline and ranges in volume from 80 to 125 cc. In women with a history of chest wall radiation, the device is placed in the prepectoral position; whereas in women without a history of radiation therapy the device is placed is the subpectoral position. Figures 17 and 18 illustrate a woman that had small implants placed beneath the flaps to augment volume.

The non-reconstructed breast
In unilateral reconstructions, the non-reconstructed breast is sometimes modified to achieve symmetry (22). Options include unilateral augmentation with an implant, mastopexy, and reduction mammaplasty. Implant selections is facilitated by volumetric analysis using 3-dimensional imaging. The technique is essentially that of a standard breast augmentation. When volumes are similar but the natural breast is ptotic, a mastopexy is often performed. This is usually via a circumvertical approach; however, when extreme, inverted T techniques are performed. When the reconstructed breast is smaller than the natural breast, reduction mammaplasty is usually performed. This can be performed using a variety of techniques that include short scars when the difference is mild to moderate and inverted T scars when the difference is moderate to severe. Figures 19 and 20 illustrate a woman following a symmetry procedure with a circumvertical reduction mammaplasty.
Conclusions
Achieving ideal breast aesthetics in the setting of autologous breast reconstruction requires artistic and technical expertise. Adherence to the basic tenants of the footprint, conus, and skin envelope as described by Blondeel et al. are essential. There are several autologous options that include pedicle flaps as well as free tissue transfers. Each flap has its unique characteristics that make it ideal or less ideal for a particular patient. Understanding the nuances of each flap is important when striving for ideal volume, contour and symmetry.
Acknowledgments
Disclosure: The author declares no conflict of interest.
References
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg 2009;123:455-62. [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Part II--Breast reconstruction after total mastectomy. Plast Reconstr Surg 2009;123:794-805. [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Part III--reconstruction following breast conservative treatment. Plast Reconstr Surg 2009;124:28-38. [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Part IV--aesthetic breast surgery. Plast Reconstr Surg 2009;124:372-82. [PubMed]
- Nahabedian MY. Breast reconstruction: a review and rationale for patient selection. Plast Reconstr Surg 2009;124:55-62. [PubMed]
- Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2012;130:282-92. [PubMed]
- Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg 2004;113:1617-28. [PubMed]
- Nahabedian MY, Momen B, Galdino G, et al. Breast Reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002;110:466-75; discussion 476-7.[PubMed]
- Nahabedian MY, Schwartz J. Autologous breast reconstruction following mastectomy. Handchir Mikrochir Plast Chir 2008;40:248-54. [PubMed]
- Chiaramonte MF, Nahabedian MY. Bilateral breast reconstruction with the latissimus dorsi musculocutaneous flap: the importance of patient positioning. Ann Plast Surg 2001;46:163-6. [PubMed]
- Heitmann C, Pelzer M, Kuentscher M, et al. The extended latissimus dorsi flap revisited. Plast Reconstr Surg 2003;111:1697-701. [PubMed]
- Hammond DC. Latissimus dorsi flap breast reconstruction. Plast Reconstr Surg 2009;124:1055-63. [PubMed]
- LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg 2010;126:393-401. [PubMed]
- Gurunluoglu R, Spanio S, Rainer C, et al. Skin expansion before breast reconstruction with the superior gluteal artery perforator flap improves aesthetic outcome. Ann Plast Surg 2003;50:475-9. [PubMed]
- Kronowitz SJ. Redesigned gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg 2008;121:728-34. [PubMed]
- Vega SJ, Sandeen SN, Bossert RP, et al. Gracilis myocutaneous free flap in autologous breast reconstruction. Plast Reconstr Surg 2009;124:1400-9. [PubMed]
- Fansa H, Schirmer S, Warnecke IC, et al. The transverse myocutaneous gracilis muscle flap: a fast and reliable method for breast reconstruction. Plast Reconstr Surg 2008;122:1326-33. [PubMed]
- Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg 2008;122:29-38. [PubMed]
- Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [PubMed]
- Nahabedian MY. Symmetrical breast reconstruction: analysis of secondary procedures after reconstruction with implants and autologous tissue. Plast Reconstr Surg 2005;115:257-60. [PubMed]
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409-22; discussion 1423-4. [PubMed]
- Nahabedian MY. Managing the opposite breast: contralateral symmetry procedures. Cancer J 2008;14:258-63. [PubMed]