
Evaluation of the diagnostic performance of contrast-enhanced ultrasound combined with BRAF V600E gene detection in nodules of unclear significance by thyroid fine-needle aspiration
Introduction
Thyroid cancer is one of the most common endocrine malignancies. The SEER database shows that its incidence has increased rapidly in recent years (1). Thyroid cancer can be divided into papillary cancer, follicular cancer, medullary cancer, and undifferentiated cancer on the pathological type. Among them, papillary thyroid cancer (PTC) is the primary type of thyroid cancer, accounting for 80–90% of thyroid malignancies (2,3). With early diagnosis and surgical treatment, the 5-year survival rate can reach 90% (4). Therefore, many scholars have proposed there is excessive diagnosis and treatment for thyroid cancer, most of which may not cause a threat to people’s lives (5-7) and only a small part of which may have extraglandular invasion, central or lateral cervical lymph node metastasis or even distant metastasis. At present, ultrasound-guided thyroid fine-needle aspiration (FNA) is the most common and effective method for the diagnosis of thyroid nodules. In 2017, the Bethesda System for Reporting Thyroid Cytopathology II (TBSRTC) (8) issued by the National Cancer Institute (NCI) standardized the interpretation of FNA results. However, because of the limitations in detection methods and techniques, some thyroid nodules still cannot be diagnosed by FNA (9). Considering thyroid malignancies, especially PTC, with a high mutation rate in the BRAF V600E gene, BRAF V600E gene detection with FNA can assist in the diagnosis of benign and malignant thyroid nodules. Moreover, contrast-enhanced ultrasound (CEUS) has been a heated topic in medical research in recent years. This technique can supply microvascular information that cannot be reflected by conventional ultrasound and observe the enhancement mode of nodules. In recent years, CEUS has been widely applied in the clinic. However, a combination of these two techniques has not been reported for unclear significance nodule evaluation by FNA. Therefore, this study aims to evaluate the diagnostic performance of CEUS combined with BRAF V600E gene detection in nodules of unclear significance by ultrasound-guided thyroid FNA.
We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/gs-20-705).
Methods
Subjects
Two hundred forty-four nodules were selected from 244 patients with nodules of unclear significance by FNA and surgical treatment who underwent CEUS and BRAF V600E detection in Lishui Hospital of Zhejiang University from January 2015 to December 2019. There were 197 females and 47 males, aged 18–80 years (45.52±10.79 years), and the largest diameter of the nodules was 2.00–51.00 mm (9.26±8.98 mm). The inclusion criteria were as follows: (I) age ≥18 years, but no limitation in gender; (II) CEUS, FNA, and BRAF V600E were detected within eight weeks before surgery, and the pathological results of FNA showed atypical lesions of unclear significance; (III) complete clinical data, imaging data, and laboratory data; and (IV) first surgical treatment and confirmation by pathology. The exclusion criteria are as follows: (I) age <18 years; (II) pregnant women; and (III) other malignant tumors.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Lishui Hospital of Zhejiang University School of Medicine approved the present study, and all subjects supplied written informed consent before their examinations.
Instruments and methods
CEUS
A color Doppler ultrasound diagnostic instrument and the high-frequency linear array transducer L12-3 enabled contrast pulse sequence (CPS) imaging, with a mechanical index of 0.08. The system supplied a dual-mode display interface. SonoVue (Bracco, Italy) was used as the contrast agent. A bottle of freeze-dried powder was dissolved by adding 5 mL of 0.9% sodium chloride solution, which was shaken sufficiently and mixed well to prepare a suspension holding 45 µg/mL sulfur hexafluoride. After the microbubbles were evenly mixed and dispersed by shaking forcefully, 2.4 mL of contrast medium suspension was suctioned into an injector and at once injected into the superficial vein of the elbow. Then, 5 mL of 0.9% NaCl solution was injected to flush the vein, and continuous dynamic images were stored synchronously for 3–5 min. The echo changes and lesion microbubble distribution were observed before and after the injection of the contrast agent. The positive indexes were as follows: (I) ring enhancement was not visible or interrupted; (II) the boundaries of the enhancement were unclear; (III) the enhancement of the nodules began later than that of the peripheral thyroid parenchymal; (IV) the clearance time of the nodules was earlier than the disappearance time of the surrounding thyroid parenchymal; (V) the enhancement of the lesions was inhomogeneous; (VI) the peak intensity of the nodules was hyperechoic or isoechoic compared with the surrounding thyroid parenchymal (10,11). We invited two attending doctors to read and score according to the positive indicators. The CEUS score was the sum of the above six indexes, and each positive item was 1, which could be accumulated.
BRAF V600E gene detection
The patients were in a supine position with the neck fully exposed. After disinfection and covering with a sterile towel and local anesthesia by subcutaneous injection with 2% lidocaine, the puncture needle was inserted into the target nodule under the guidance of ultrasound. Five repeated aspirations were performed in different directions, and FNA samples were injected into the Thinprep solution and sent to the Department of Pathology. The puncture samples were added to the gene detection kit (ADx-BR02) provided by Amoy Diagnostics Co., Ltd. (Xiamen), followed by DNA extraction and polymerase chain reaction (PCR) amplification (real-time fluorescence quantitative PCR-ARMS was used for detection). The results were analyzed according to the instructions, and mutations in the BRAF V600E gene were obtained. The presence of the mutation was scored as 1, and the absence of the mutation was scored as 0.
Combined diagnostic criteria were used in this study. With pathological results as the gold standard, the diagnostic value of CEUS, BRAF V600E gene mutation detection, and the combined application in nodules of unclear significance by thyroid FNA was evaluated and compared by adding the scores of CEUS and BRAF V600E gene mutation detection.
Statistical methods
Statistical analysis was performed by SPSS 23.0. The measurement data with a normal distribution are expressed as the mean ± standard deviation and are analyzed by the group design t-test. The enumeration data were compared using the χ2 test. The sensitivity, specificity, PPV, NPV, and accuracy of CEUS, BRAF V600E gene detection, and the combination in the diagnosis of benign and malignant nodules were calculated, with postoperative pathological results as the gold standard. Additionally, a ROC curve was constructed, the area under the ROC curve (AUC) was calculated and compared, and the optimal diagnostic boundary value of the benign and malignant nodules was determined. P<0.05 was considered statistically significant.
Results
Surgical and pathological results
A total of 244 nodules of unclear pathological significance by FNA were obtained, and routine pathological reports were obtained after surgery. There were 205 malignant nodules, including 203 cases of PTC, 1 case of medullary thyroid cancer, and 1 case of follicular thyroid cancer. In the benign nodules, there are 18 cases of nodular goiter, 13 cases of Hashimoto’s thyroiditis, 5 cases of thyroid adenoma, and 3 cases of granulomatous inflammation.
Comparison of CEUS with the pathological results
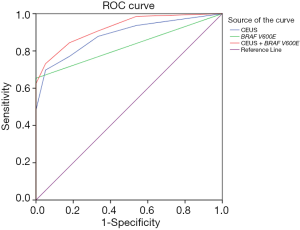
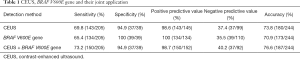
After injection of the contrast agent into 205 cases of malignant nodules, 125 malignant nodules presented low inhomogeneous enhancement with unclear boundaries, 53 showed centripetal inhomogeneous peripheral enhancement with punctate enhancement in the center, and 27 exhibited homogeneous equal or high enhancement. Among the 39 benign nodules, 29 benign nodules presented homogeneous equal or high enhancement (high ring enhancement was found in 23 benign nodules), and the other 10 showed low enhancement or inhomogeneous enhancement. According to different manifestations, scoring was conducted, and ROC curves were drawn. The optimal threshold score of CEUS in the differential diagnosis of thyroid nodules of unclear significance by FNA was 3 (AUC =0.884, Figure 1). Its sensitivity, specificity, PPV, NPV, accuracy and AUC were 69.8%, 94.9%, 98.6%, 37.4%, 73.8% and 0.884, respectively.
Comparison of BRAF V600E gene detection with the pathological results
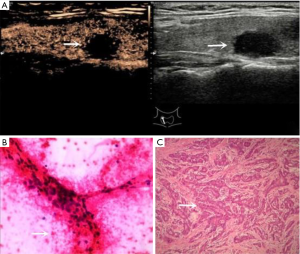
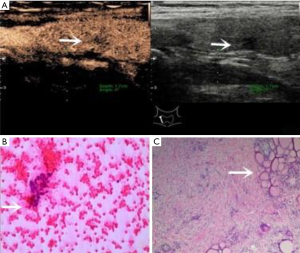
Among the 244 nodules, 134 were BRAF V600E-positive and were all confirmed as PTC by pathological results. Of the 110 nodules that were BRAF V600E-negative, 71 were confirmed as PTC (Figure 2), one as medullary thyroid cancer, one as follicular thyroid cancer, 18 as nodular goiter (Figure 3), 13 as Hashimoto’s thyroiditis, 5 as thyroid adenomas and 3 as granulomatous inflammation. With the BRAF V600E mutation as the positive standard, the sensitivity, specificity, PPV, NPV, accuracy, and AUC in diagnosing thyroid nodules of unclear significance by FNA were 65.4%, 100%, 100%, 35.5%, 70.9% and 0.827, respectively.
CEUS combined with BRAF V600E gene mutation detection results
The sensitivity, specificity, PPV, NPV, accuracy, and AUC of CEUS combined with BRAF V600E gene mutation detection in diagnosing thyroid nodules of unclear significance by FNA were 73.2%, 94.9%, 98.7%, 40.2%, 76.6% and 0.923, respectively. ROC curve analysis showed that the optimal threshold of their combination in diagnosis was 3 (AUC =0.923). The sensitivity, NPV, and accuracy of the combined application in the diagnosis of thyroid nodules of unclear significance by FNA were significantly higher than those of the single applications (P<0.001) (Tables 1,2; Figure 1).
Full table

Full table
Discussion
Thyroid cancer has increased rapidly in the last few years (1). At present, the preoperative diagnosis of PTC mainly depends on high-frequency ultrasound and FNA (12,13). Color Doppler ultrasound and elastic imaging can evaluate the benignancy and malignancy of nodules by semiquantitative analysis of differences in tissue hardness. These techniques have high diagnostic value for PTC and cervical lymph node metastasis, with fibrosis in lesions as the main change. The diagnostic accuracy of PTC is 74–82%. Also, FNAB is a critical preoperative diagnostic method for PTC. More than 80% of lesions can be diagnosed by observing the characteristics of cell morphology under a microscope (14,15). However, due to the limitations in examination methods and techniques and insufficient specificity of cytology and morphology in some PTCs, there are still difficulties in the preoperative diagnosis of some thyroid nodules, which has made it difficult to improve the accuracy of the preoperative diagnosis of PTC. Although repeated FNA is recommended in the 2nd edition of the Guidelines for Thyroid Cell Pathology Report System for nodules of unclear significance, up to 50% of these repeated FNA analyses result in missed diagnosis due to insufficient cells or other causes. Moreover, surgical resection is also suggested, which leads to the overtreatment of thyroid nodules.
CEUS is a novel noninvasive imaging technology developed in recent years and is also a hot topic in current medical research. This technique can display the microvasculature in nodules with diameters <10 µm by intravenous injection of a microbubble contrast agent combined with ultrasonic pulse inversion harmonic imaging. Then, the technique allows for the evaluation of the characteristics of intranodal perfusion and provides a corresponding diagnosis by observing the differences in perfusion patterns between benign and malignant nodules. These factors make it possible to quantitatively analyze the state of microvascular perfusion with a time-intensity curve (16), which allows for the investigation of microcirculatory perfusion in tumors. In this study, malignant nodules accounted for 84.02% (205/244), of which 86.83% (178/205) presented inhomogeneous low enhancement and slow-forward and fast-backward substantial enhancement, which is consistent with the results reported by Ma et al. (17). Another 27 cases show homogeneous equal or high enhancement, which may be related to the mild infiltration of neovascularization and small perforating vessels in the tumor and the low tumor volume. Also, 25.64% (10/39) of the benign nodules in this study showed low inhomogeneous enhancement, which may be related to the histological characteristics of the nodules themselves. There were recurrent hemorrhagic cystic changes and repeated hyperplasia of fibrous tissue or accompanied by coarse calcification, which led to inhomogeneous enhancement in imaging (18,19).
With the continually deepening exploration of tumor pathogenesis and biological behavior, increasing reports on chromosomes and specific molecular markers related to PTC pathogenesis have been found (20-23). The current research results show that PTC-specific genetic events include the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which initiates a cascade reaction leading to tumorigenesis (22,23). Mutations or rearrangements of oncogenes in the RET/PTC, BRAF and RAS families cause the activation of tyrosine kinase and related pathways, which are the early and independent activators of the MAPK signaling pathway in PTC and have a significant impact on the transformation of thyroid follicular cells into PTC (23). Among them, BRAF V600E gene mutations are the most common (22). Some scholars even believe its occurrence is related to the prognosis of PTC (21-23). In this study, the sensitivity and specificity of BRAF V600E gene detection in the diagnosis of benign and malignant thyroid nodules were 65.4% and 100%, respectively, which is consistent with the conclusion of Rodrigues et al. (24) that this gene has high specificity and low sensitivity.
Currently, reports about the correlation between CEUS of PTC and the expression of pathological molecular markers are lacking at home and abroad. In this study, the correlation between the perfusion characteristics of PTC in ultrasound microvascular imaging and molecular markers was analyzed to improve the diagnosis further, provide a theoretical basis, and determine the clinical value. After comparing the results, we found that the combination of CEUS and BRAF V600E detection could improve the sensitivity, NPV, and accuracy of the diagnosis, suggesting that the combination reduced false negative and positive pathological results of CEUS or BRAF V600E gene detection alone. Second, ROC curve analysis revealed that the AUC of CEUS combined with BRAF V600E gene detection was significantly larger than single-use. When the diagnostic score was 3, the sensitivity and specificity were ideal. Therefore, we believe that a CEUS score of 3 can be regarded as the optimal quantitative score, that is, a score <3 is benign, and one ≥3 is malignant. However, the sample size of this study is inadequate, so the correct optimal boundary value needs to be further verified by expanding the sample size.
Acknowledgments
Funding: Lishui Science and Technology Bureau Self-Raised Public Welfare Technology Application Project supported the present study in 2019 (Fund No. 2019SJZC44).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-705
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-705
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-705). All authors report grants from Lishui Science and Technology Bureau, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Lishui Hospital of Zhejiang University School of Medicine approved the present study, and all subjects supplied written informed consent before their examinations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang Y, Wang W. Increasing Incidence of Thyroid Cancer in Shanghai, China, 1983-2007. Asia Pac J Public Health 2015;27:NP223-9. [Crossref] [PubMed]
- Nix P, Nicolaides A, Coatesworth AP. Thyroid cancer review I: presentation and investigation of thyroid cancer. Int J Clin Pract 2005;59:1340-4. [Crossref] [PubMed]
- Zhang TT, Qi XZ, Chen JP, et al. The association between tumor's location and cervical lymph nodes metastasis in papillary thyroid cancer. Gland Surg 2019;8:557-68. [Crossref] [PubMed]
- Zimmerman D, Hay ID, Gough IR, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988;104:1157-66. [PubMed]
- Cronan JJ. Thyroid nodules: Is it time to turn off the US machines? Radiology 2008;247:602-4. [Crossref] [PubMed]
- Li R, Wang Y, Du L.. A rapidly increasing trend of thyroid cancer incidence in selected East Asian countries: Joinpoint regression and age-period-cohort analyses. Gland Surg 2020;9:968-84. [Crossref] [PubMed]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic The increasing impact of overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Ali SZ, Cilbas ES. The Bethesda system for reporting thyroid cytopathology II. Acta Cytol 2016;60:397-8. [Crossref] [PubMed]
- Seshadri KG. A pragmatic approach to the indeterminate thyroid nodule. Indian J Endocrinol Metab 2017;21:751-7. [Crossref] [PubMed]
- Wu Q, Wang Y, Li Y, et al. Diagnostic value of contrast-enhanced ultrasound in solod thyroid nodules with and without enhancement. Endocrine 2016;53:480-8. [Crossref] [PubMed]
- Chen HY, Liu WY, Zhu H, et al. Diagnostic value of contrast-enhanced ultrasound in papillary thyroid microcarcinoma. Exp Ther Med 2016;11:1555-62. [Crossref] [PubMed]
- Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003;63:1454-7. [PubMed]
- Shaha AR, Tuttle RM. Thyroid cancer staging and genomics. Ann Transl Med 2019;7:S49. [Crossref] [PubMed]
- Soares P, Trovisco V, Rocha AS, et al. BRAF Mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003;22:4578-80. [Crossref] [PubMed]
- Xu X, Qurios RM, Gattuso P, et al. High prevalence of BRAF gene mutation in papillay thyroid carcinoma and thyroid tumor cell lines. Cancer Res 2003;63:4561-7. [PubMed]
- Zhang B, Jiang YX, Liu JB, et al. Utility of Contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid 2010;20:51-7. [Crossref] [PubMed]
- Ma HJ, Yang JC, Leng ZP, et al. Preoperative prediction of papillary thyroid microcarcinoma via multiparameter ultrasound. Acta Radiol 2017;58:1303-11. [Crossref] [PubMed]
- Rubaltelli L, Corradin S, Dorigo A, et al. Differential diagnosis of benign and malignant thyroid nodules at elastosonography. Ultraschall Med 2009;30:175-9. [Crossref] [PubMed]
- Cantisani V, D'Andrea V, Mancuso E, et al. Prospective evaluation in 123 patients of strain ratio as provided by quantitative elastosonography and multiparametric ultrasound evaluation (ultrasound score) for the characterisation of thyroid nodules. Radiol Med 2013;118:1011-21. [Crossref] [PubMed]
- Rodrigues R, Roque L, Espadinha C, et al. Comparative genomic hybridization, BRAF, RAS, RET, and oligo-array analysis in aneuploid papillary thyroid carcinomas. Oncol Rep 2007;18:917-26. [Crossref] [PubMed]
- Vu-Phan D, Koenig RJ. Genetics and epigenetics of sporadic thyroid cancer. Mol Cell Endocrinol 2014;386:55-66. [Crossref] [PubMed]
- Xing M, Westra WH, Tufano RP. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90:6373-9. [Crossref] [PubMed]
- Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic thyroid carcinomas. J Clin Endocrinol Metab 2004;89:2414-20. [Crossref] [PubMed]
- Rodrigues HG, de Pontes AA, Adam LF. Use of molecular markers in sample obtained from preoperative aspiration of thyroid. Endocr J 2012;59:417-24. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


