Oncoplastic breast surgery: current strategies
Introduction
Surgical management of breast cancer has evolved significantly over the years, trending away from radical procedures, and moving towards those with complete resection of tumor while preserving normal parenchyma tissue thereby decreasing patient morbidity. This shift has allowed for improved aesthetic outcomes and quality-of-life for patients, while maintaining equivalent oncologic safety (1,2).
A more recent innovation to further enhance aesthetic outcomes has been the development of “oncoplastic” surgery, which broadly refers to reconstruction of partial mastectomy defects. A variety of techniques have been described for partial mastectomy reconstruction, including local tissue rearrangement, reconstruction through reduction mammoplasty or mastopexy approaches, and transfer of local-regional flaps.
The rapidly expanding body of literature on outcomes following oncoplastic surgery has shown numerous benefits to this reconstructive approach, including improved aesthetic outcomes (3,4), better control of tumor margins (5), high patient satisfaction (6-8), and the ability to extend the option of breast conservation (9-11).
This review will describe a comprehensive approach to evaluating and treating patients with oncoplastic reconstruction as well as summarize the different approaches and outcomes for the various techniques.
Pre-operative evaluation
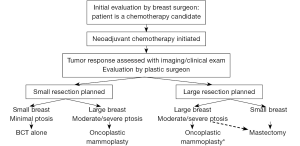
In the patient who is a candidate for oncoplastic breast surgery, it is necessary to have a multidisciplinary preoperative evaluation with the breast oncologic surgeon and plastic surgeon. The breast oncologic surgeon will determine the volume and location of breast to be resected thereby providing information as to the anticipated defect that will be reconstructed, and whether or not the patient is a candidate for breast conservation therapy. Preoperative planning affords surgeons increased flexibility in terms of incision design and pedicle selection. Some patients with locally advanced breast cancer may be candidates for neoadjuvant chemotherapy. Tumor shrinkage through this preoperative treatment, resulting in tumor downstaging, may then allow these patients to become candidates for breast conserving surgery (12-16). The option of significant tissue rearrangement through oncoplastic techniques can facilitate the removal of larger tumors, which can potentially extend the option of breast conservation to patients who would have traditionally required mastectomy (9). It is particularly important to consider the combination of neoadjuvant chemotherapy for tumor shrinkage followed by oncoplastic surgery in patients who will require post-operative radiation therapy even if they have a mastectomy, given the high rates of complications following post-mastectomy breast reconstruction and subsequent post-mastectomy radiation therapy (11,17). It is also important to establish expectations both of the patient and the surgeons during the preoperative period.
The preoperative evaluation should include examination for degree of ptosis, overall skin quality, evidence of prior radiation, and overall breast size. The reconstructive options available are primarily determined by the size of the breast and the tumor to breast ratio. In the smaller breasted woman, there is less glandular tissue available to perform local tissue rearrangement, and therefore these patients are more likely to need regionally-based flaps. Mastectomy with reconstruction may provide a more aesthetically pleasing result than breast conservation surgery in the small to moderate-breasted woman with a large tumor (on average, a resection size to breast size ratio greater than 1:5). Larger breasted women have more options available for reconstruction, whether it is local tissue rearrangement, local or regional flaps, or reduction mammoplasty/mastopexy. In the oncoplastic breast reduction, tumor location will dictate the reduction technique used and the design of the nipple/areolar pedicle.
Given that the majority of women with breast cancer are older than 50, and with aging there is inferolateral descent of the breast and nipple-areolar complex (NAC), there will often be contralateral breast asymmetry following resection and reconstruction of the affected breast. Many women desire symmetry-achieving surgery following oncoplastic breast surgery. Both breasts play equal roles in the “aesthetic triangle”, therefore the contralateral breast’s appearance is vital in the overall aesthetic outcome. Relocation of the NAC and achieving volumetric symmetry greatly improve the overall result. However, controversy exists over timing of symmetry-achieving surgery. Some institutions perform synchronous surgery with the affected breast, while others delay symmetry surgery given the potential effects of hormonal therapy, chemotherapy and radiation therapy on morbidity, and on further changing the shape and appearance of the effected breast (18-20). There have been reports as to the timing of these procedures in post-mastectomy reconstruction, with excellent aesthetic outcomes reported for synchronous procedures.
Furthermore, several studies have reported uncovering occult malignancies in the contralateral breast, with an overall rate ranging from 0.16-5% (21-24). Additionally, there is evidence that breast reduction significantly reduces breast cancer incidence in women over the age of 50 (25). Therefore, the benefits of symmetry surgery on the non-disease breast may be more than just producing an improved aesthetic outcome.
Oncoplastic techniques
Oncoplastic breast surgery entails complete tumor extirpation, partial reconstruction of wide local excisions, and symmetrizing surgery for the contralateral breast (26). The technique used for reconstruction depends on a number of factors, most importantly tumor location and size, tumor to breast size ratio, and patient desires.
Local tissue rearrangement
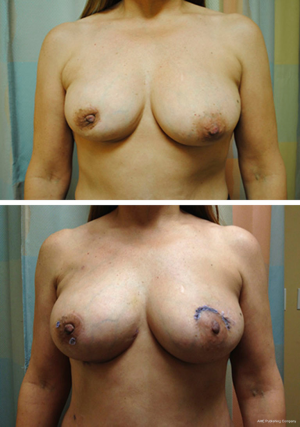
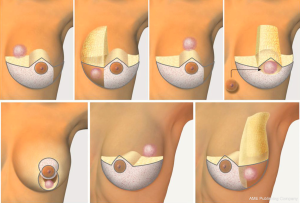
Local tissue rearrangement is an essential component of many oncoplastic techniques. It is most commonly used in women with moderate-sized breasts, small tumors and grade 1 ptosis. This technique may shift the defect to a less conspicuous location by taking advantage of subcutaneous fat and skin elsewhere (see Figure 1). These approaches often involve raising of skin/subcutaneous flaps to allow for mobilization of the underlying glandular tissue to fill the glandular defect. Glandular flaps may allow defects in all areas of the breast to be filled, even in the difficult-to-repair upper inner quadrant defects, provided there is sufficient tissue (see Figure 2).
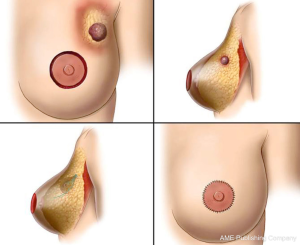
If there is insufficient tissue for local tissue rearrangement because the defect is too large, local or regional flaps provide viable options for reconstruction. Local flaps from the subaxillary region are useful for moderate defects in the smaller breast. More lateral defects may be reconstructed with a transposition or rotational flap, moving skin and subcutaneous fat that is lateral to the breast (28) into defects in the outer quadrants of the breast. The latissimus dorsi flap provides enough volume to correct almost any partial mastectomy defect, is technically simple and has relatively low morbidity (29,30). Because of the different skin color and texture with this flap, it is better to replace an entire aesthetic unit during latissimus dorsi reconstruction. This is ideally done by having one edge of the skin paddle form the inframammary fold, the lateral breast border, or both (28). However, this flap can still be used if no skin is missing by transferring the muscle alone.
Mastopexy approaches
Mastopexy techniques are good options for patients with significant ptosis and adequate breast volume, as well as larger breasted patients (31). These procedures, in conjunction with partial mastectomy, help maintain an aesthetically pleasing breast shape following large tumor resections (see Figure 3). Benelli’s ‘round block’ technique is ideal for upper pole tumors close to the areola in mildly ptotic breasts that would benefit from mastopexy (32). This technique involves de-epithelialization of the peri-areolar area with the NAC supplied by a central glandular pedicle. Local parenchymal remodeling with wide skin undermining is performed after tumor excision. The same technique may be used on the contralateral breast at the same time or following radiotherapy to achieve symmetry (33). The omega-plasty, or ‘batwing’ mastopexy is another good option for tumors of the upper pole (31). It involves wide en bloc resection of superior peri-areolar skin, gland and tumor to the pre-pectoral plane, with the shape of the final resected skin and glandular specimen having a ‘batwing’ type appearance. Wound closure is performed in a layered fashion, which allows for elevation of the inferior quadrants and NAC, thereby correcting ptosis (33).
Oncoplastic reduction mammoplasty
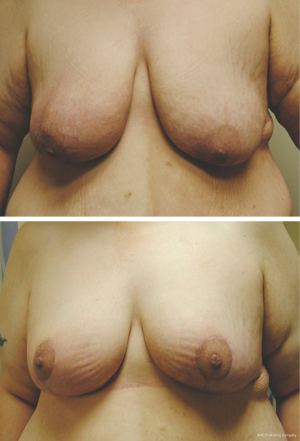
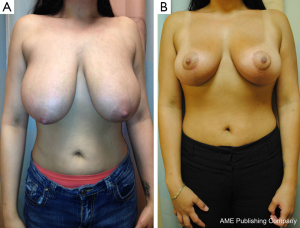
Bilateral reduction mammoplasty is an ideal treatment option for breast cancer in women with preoperative macromastia (21,34,35). Based on tumor location, a skin pattern and NAC pedicle are designed pre-operatively to allow for resection of the tumor within the typical resection pattern for the specific reduction technique chosen, and filling of the planned tumor defect with the remaining breast tissue (see Figures 4 and 5). Once the amount of required tissue resection is determined on the ipsilateral side, the contralateral breast is reduced to match (36). This technique can also be applied to tumors in other areas of the breast by shifting tissue and rotating the reduction pattern (28).
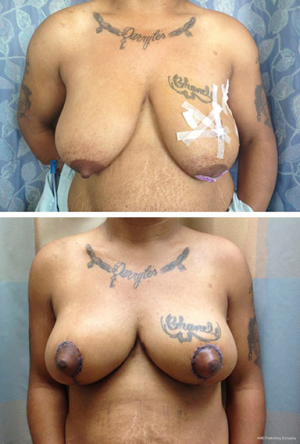
The most commonly employed oncoplastic technique is the Wise pattern with inferior pedicle reduction mammoplasty (33). This technique combines wide upper pole tumor excision with excess gland resection, resulting in an improved aesthetic for the large or ptotic breast. The incision pattern maintains viability of the skin flaps while providing adequate access and exposure for the partial mastectomy to be performed. The dermo-glandular pedicle vascularizes the NAC, thus keeping it well perfused and viable. This technique can also be used for peri-areolar and central tumors.
The vertical scar technique is ideal for inferior pole tumors and central subareolar tumors as they may be widely excised within the boundaries of the standard markings. It was first described by Lassus (37), then popularized by Lejour (38) for aesthetic breast surgery. The advantages of this technique include shorter skin incisions, straightforward glandular resection, and a shorter pedicle which offers reliable blood supply to the NAC for a variety of breast sizes (39). Use of this approach in oncoplastic reconstruction has become increasingly popular, with recent studies demonstrating good cosmetic and oncologic outcomes, and high patient satisfaction (6).
Lateral pole tumors are well suited for lateral mammoplasty. This technique combines wide tumor excision with supero-medial NAC repositioning on a dermo-glandular pedicle, thereby counteracting lateral axial scar contraction and breast ptosis. Good or excellent outcomes have been reported in the majority of reconstructions performed with this technique (40). Additionally, the incision may be extended superiorly to access the axilla for node dissections without having to make a separate incision. Medial mammoplasty, used for medial tumors, is almost the mirror image of lateral mammoplasty. The NAC pedicle is de-epithelialized to allow its repositioning on the breast mound. For larger volume resections, extending the incision along the medial IMF allows for parenchymal rotation flaps to be used.
Oncoplastic reconstructive algorithm
A number of studies have been published describing oncoplastic technique algorithms based on tumor location (see Figure 6) (27). Berry et al. described ten oncoplastic techniques based on tumor location (33). In a similar manner, Iwuchukhu et al. divided the breast into seven zones (see Figure 7), and each zone corresponded with several suggested mammoplasty techniques (41). Overall, tumor location can be divided into the upper or lower pole, and then whether it lies medially, laterally or centrally.
The majority of breast cancers are found in the upper outer or lower outer quadrants. Most of these tumors may be treated with the inferior pedicle technique (42,43), which is the most common form of breast reduction. This technique allows for removal of additional breast tissue, maintains nipple perfusion, and achieves an aesthetic and symmetric reconstruction. Upper outer tumors can also be treated using a superior-medial extended pedicle through a Wise incision (44,45).
Lower pole tumors can be excised using a superior or superior-medial based pedicle using the Wise pattern skin envelope or vertical mammoplasty technique. Good or very good cosmetic outcomes have been reported in the majority of these patients (46-48).
Upper pole tumors are more difficult to reconstruct given the difficulty of maintaining upper pole breast volume following wide local excision. The inferior pedicle approach (44), round block technique (49) and “batwing” design (31) are all suitable techniques. Tumors of the upper inner quadrant are especially difficult to reconstruct given their more visible location post-operatively. Various approaches have been reported, including an extended superior-lateral pedicle (35,50), extended inferior pole pedicle that would normally be discarded as part of the reduction mammoplasty (28,42), and lateral pedicle with up-rotation of the whole breast (51) all with good cosmetic results.
Medial tumors can be easily access via a Wise pattern skin incision with an extended superior pedicle flap. A supero-lateral nipple pedicle can be extended inferiorly and then rotating the inferior pole upwards to fill the defect, thereby negating the increased risk of fat necrosis associated with two pedicles. Local rotation of breast parenchyma is also suitable for this zone (44).
Lateral tumors can be resected via a Wise incision or inverted “T” pattern incision and filled using a superior-medial pedicle (52). The Wise pattern skin incision affords better access for tumor resection, as well as allows for axillary surgery through the tail of the incision (44). Several other techniques have been described to repair this defect, including rotation of adjacent breast tissue (32), lateral thoracic rotation flap (53,54), latissimus dorsi myocutaneous rotational flap (28) and matrix rotation flap (55).
Central tumors present a unique challenge in that they may or may not require resection of the NAC. If the nipple is left in place, a standard Wise or vertical mammoplasty incision can be used with an inferior, medial or lateral pedicle. Fitzal et al. suggested that a medio-inferior pedicle technique may preserve nipple sensation better than either superior or inferior pedicles (51). A simple approach is excision via the inverted “T” closing wedge or melon slice mammoplasty, which does not require a planned NAC pedicle (56). Therefore, the risk of fat necrosis and pedicle necrosis are decreased making this a more appealing option for high risk patients. If NAC removal is required, a Wise pattern incision with an inferior pedicle to fill the central defect has been demonstrated to have good outcomes (57). Nipple reconstruction can either be performed at the time of initial reconstruction, or delayed. Options for immediate nipple reconstruction include creation on an advanced skin paddle (44), as well as reconstruction using a full thickness skin graft (58).
Outcomes
Complications
Overall complication rates for oncoplastic reconstruction range from 15-30% and have been well-documented (11,59-61). The complications unique to this type of surgery include skin/flap necrosis, nipple and nipple areola complex necrosis, seroma, hematoma, infection, wound dehiscence and fat necrosis. The most common complication in Wise pattern/inverted “T” techniques is delayed healing of the “T” junctions (the areas where perpendicular scars meet. This is due to reduced vascular perfusion. While wound healing complications may delay time to adjuvant radiotherapy, this is a rare occurrence in all series reported to date. These procedures do have longer operating times than wide local excision alone, which should be taken into consideration when evaluating patients to ensure they are appropriate candidates for oncoplastic reconstruction.
Oncologic outcomes
Recurrence
With oncoplastic reconstruction, concern exists that local tissue rearrangement may impact local recurrences and the ability to detect them. However, numerous studies have demonstrated that oncoplastic techniques have low local recurrence rates when compared with breast conserving therapy alone (62). Reitjens et al. found that local recurrence rates were low over long-term follow-up, with a 3% rate at 5 years and no recurrences seen in those tumors smaller than 2 cm (24). This was echoed by Caruso et al., who reported a 1.5% local recurrence rate when evaluating 63 women who had undergone bilateral reduction mammoplasty (43). This was similar to the 2.5% recurrence rate Chang et al. found in an evaluation of 79 patients who underwent simultaneous partial mastectomy and reduction mammoplasty (9). In a prospective cohort study of patients with locally advanced breast cancer undergoing oncoplastic surgery, Bogusevicius et al. reported a local regional recurrence rate of 10%, at 86 months (63). However, these patients had larger tumors and longer follow-up than the previously mentioned studies. Additionally, excision of multifocal tumors within the same quadrant has been shown to be oncologically safe with the wide margins that can be taken with oncoplastic procedures (64).
Positive margins
While oncoplastic techniques allow for wider resections, the tissue rearrangement performed in reconstruction may complicate management of positive margins. Positive margins have been reported between 2.7-22% (9,20,62,63) and have been associated with higher stage, positive nodes, positive lymphovascular invasion, use of neoadjuvant chemotherapy, larger initial “T” stage, positive estrogen receptor and younger age (20,51,65). Many oncoplastic techniques utilize dermo-glandular rotational flaps, which transpose tissue from one area of the breast to another. If a second surgical stage is needed for presence of disease at the edges of the specimen, this can become challenging due to the displacement of the glandular tissue, thereby making further excision very difficult (41). Although re-excision is possible, more often these patients undergo completion mastectomy. Additionally, since most mammoplasty techniques rely on a unipedicle or bipedicle, subsequent need for surgery risks pedicle compromise thereby restricting future therapeutic options. However, it has been demonstrated that patients undergoing oncoplastic surgery are more likely to have negative margins compared to partial mastectomy alone (5,66). This is likely due to the more aggressive resection afforded to the surgical oncologist, with the knowledge that the oncoplastic reduction will limit the aesthetic detriment following this procedure. Giacalone et al. found that patients who underwent oncoplastic surgery were more likely to achieve 5 or 10 mm free margins in a significantly higher percentage of cases compared with patients who underwent quadrantectomies (67).
Intraoperative frozen section has been evaluated as a means to combat positive margins. Rusby et al. used frozen section as a diagnostic technique to evaluate margins in patients undergoing latissimus dorsi mini-flaps at the time of partial mastectomy. One third of patients had positive frozen sections with a sensitivity of 83% and accuracy of 96% when compared with paraffin sections. Overall, local recurrence rate was 0.9% with a median follow-up of 41.4 months (68). Caruso et al. evaluated the utility of intraoperative frozen section in patients undergoing therapeutic mammoplasty. They found that 8/52 patients (3 false positives, 5 true positives) had positive frozen sections, with a sensitivity of 83% and accuracy of 94%. Based on their findings, they advocated for intra-operative assessment of margins as a means of improving local control in a single stage, thereby reducing the need for secondary re-excisions or mastectomies (none in their study) (69).
Need for completion mastectomy
Although large long-term follow-up studies are lacking for oncoplastic breast surgery, published studies have described low rates of completion mastectomy. Reported rates have ranged from 5% to 10% (9,21,24,33). These low rates have been demonstrated despite inclusion of patients with tumors greater than 4 cm in size (9).
Aesthetic outcomes/patient satisfaction
Overall, oncoplastic breast reconstruction results in better aesthetic outcomes and higher patient satisfaction relative to breast conserving oncologic surgery without reconstruction. Bogusevicius et al. found that 87.2% of patients had good to excellent aesthetic outcomes in patients with locally advanced breast cancer undergoing oncoplastic surgery (63). The vast majority of patients (>80%) who underwent therapeutic mammoplasty over mastectomy or lumpectomy would make the same choice if given that choice again (21,34). Veiga et al. found that patients who underwent reduction mammoplasty following partial mastectomy had improved self-esteem and mental health when compared with patients who did not undergo reconstruction following partial mastectomy (4). However, patients undertaking oncoplastic procedures have higher expectations compared with classic conservative treatment (70). This most likely explains why between 5-14% of patients undergoing oncoplastic surgery reportedly have a poor cosmetic outcome (10,21,34,35,49,50,71-73).
Conclusions
Oncoplastic breast reconstruction at the time of partial mastectomy, either through local tissue rearrangement or mastopexy/reduction mammoplasty technique, is an extremely valuable tool in comprehensive oncologic treatment. These techniques leave patients with minimal breast deformities following proper treatment, without compromising oncologic safety. These are procedures that all reconstructive breast surgeons should be familiar with and offer their patients at the time of breast conserving surgery for breast cancer.
Acknowledgments
Disclosure: The authors declare no conflict of interest.
References
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567-75. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Clough KB, Ihrai T, Oden S, et al. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg 2012;99:1389-95. [PubMed]
- Veiga DF, Veiga-Filho J, Ribeiro LM, et al. Evaluations of aesthetic outcomes of oncoplastic surgery by surgeons of different gender and specialty: a prospective controlled study. Breast 2011;20:407-12. [PubMed]
- Losken A, Pinell-White X, Hart AM, et al. The oncoplastic reduction approach to breast conservation therapy: benefits for margin control. Aesthet Surg J 2014;34:1185-91. [PubMed]
- Barnea Y, Inbal A, Barsuk D, et al. Oncoplastic reduction using the vertical scar superior-medial pedicle pattern technique for immediate partial breast reconstruction. Can J Surg 2014;57:E134-40. [PubMed]
- Chan SW, Cheung PS, Lam SH. Cosmetic outcome and percentage of breast volume excision in oncoplastic breast conserving surgery. World J Surg 2010;34:1447-52. [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [PubMed]
- Chang EI, Peled AW, Foster RD, et al. Evaluating the feasibility of extended partial mastectomy and immediate reduction mammoplasty reconstruction as an alternative to mastectomy. Ann Surg 2012;255:1151-7. [PubMed]
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [PubMed]
- Peled AW, Sbitany H, Foster RD, et al. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast J 2014;20:302-7. [PubMed]
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85. [PubMed]
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;(30):96-102.
- van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37. [PubMed]
- Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann Oncol 1999;10:47-52. [PubMed]
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007;CD005002. [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg 2014;134:396-404. [PubMed]
- Clough KB, Baruch J. Plastic surgery and conservative treatment of breast cancer. Indications and results. Ann Chir Plast Esthet 1992;37:682-92. [PubMed]
- Berry MG, Cucchiara V, Davies DM. Breast augmentation: Part II--Adverse capsular contracture. J Plast Reconstr Aesthet Surg 2010;63:2098-107. [PubMed]
- Bong J, Parker J, Clapper R, et al. Clinical series of oncoplastic mastopexy to optimize cosmesis of large-volume resections for breast conservation. Ann Surg Oncol 2010;17:3247-51. [PubMed]
- Chang E, Johnson N, Webber B, et al. Bilateral reduction mammoplasty in combination with lumpectomy for treatment of breast cancer in patients with macromastia. Am J Surg 2004;187:647-50; discussion 650-1. [PubMed]
- Kakagia D, Fragia K, Grekou A, et al. Reduction mammaplasty specimens and occult breast carcinomas. Eur J Surg Oncol 2005;31:19-21. [PubMed]
- Petit JY, Rietjens M, Contesso G, et al. Contralateral mastoplasty for breast reconstruction: a good opportunity for glandular exploration and occult carcinomas diagnosis. Ann Surg Oncol 1997;4:511-5. [PubMed]
- Rietjens M, Urban CA, Rey PC, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast 2007;16:387-95. [PubMed]
- Boice JD Jr, Persson I, Brinton LA, et al. Breast cancer following breast reduction surgery in Sweden. Plast Reconstr Surg 2000;106:755-62. [PubMed]
- Baildam AD. Oncoplastic surgery of the breast. Br J Surg 2002;89:532-3. [PubMed]
- Kronowitz SJ, Kuerer HM, Buchholz TA, et al. A management algorithm and practical oncoplastic surgical techniques for repairing partial mastectomy defects. Plast Reconstr Surg 2008;122:1631-47. [PubMed]
- Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg 1999;104:409-20. [PubMed]
- Papp C, Wechselberger G, Schoeller T. Autologous breast reconstruction after breast-conserving cancer surgery. Plast Reconstr Surg 1998;102:1932-6; discussion 1937-8.
- Slavin SA, Love SM, Sadowsky NL. Reconstruction of the radiated partial mastectomy defect with autogenous tissues. Plast Reconstr Surg 1992;90:854-65; discussion 866-9. [PubMed]
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57. [PubMed]
- Benelli L. A new periareolar mammaplasty: the "round block" technique. Aesthetic Plast Surg 1990;14:93-100. [PubMed]
- Berry MG, Fitoussi AD, Curnier A, et al. Oncoplastic breast surgery: a review and systematic approach. J Plast Reconstr Aesthet Surg 2010;63:1233-43. [PubMed]
- Newman LA, Kuerer HM, McNeese MD, et al. Reduction mammoplasty improves breast conservation therapy in patients with macromastia. Am J Surg 2001;181:215-20. [PubMed]
- Spear SL, Pelletiere CV, Wolfe AJ, et al. Experience with reduction mammaplasty combined with breast conservation therapy in the treatment of breast cancer. Plast Reconstr Surg 2003;111:1102-9. [PubMed]
- Kroll SS, Singletary SE. Repair of partial mastectomy defects. Clin Plast Surg 1998;25:303-10. [PubMed]
- Lassus C. Breast reduction: evolution of a technique--a single vertical scar. Aesthetic Plast Surg 1987;11:107-12. [PubMed]
- Lejour M. Vertical mammaplasty and liposuction of the breast. Plast Reconstr Surg 1994;94:100-14. [PubMed]
- Hall-Findlay EJ. A simplified vertical reduction mammaplasty: shortening the learning curve. Plast Reconstr Surg 1999;104:748-59. [PubMed]
- Ballester M, Berry M, Couturaud B, et al. Lateral mammaplasty reconstruction after surgery for breast cancer. Br J Surg 2009;96:1141-6. [PubMed]
- Iwuchukwu OC, Harvey JR, Dordea M, et al. The role of oncoplastic therapeutic mammoplasty in breast cancer surgery--a review. Surg Oncol 2012;21:133-41. [PubMed]
- Kronowitz SJ, Hunt KK, Kuerer HM, et al. Practical guidelines for repair of partial mastectomy defects using the breast reduction technique in patients undergoing breast conservation therapy. Plast Reconstr Surg 2007;120:1755-68. [PubMed]
- Caruso F, Catanuto G, De Meo L, et al. Outcomes of bilateral mammoplasty for early stage breast cancer. Eur J Surg Oncol 2008;34:1143-7. [PubMed]
- McCulley SJ, Macmillan RD. Planning and use of therapeutic mammoplasty--Nottingham approach. Br J Plast Surg 2005;58:889-901. [PubMed]
- Losken A, Styblo TM, Carlson GW, et al. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg 2007;59:235-42. [PubMed]
- Munhoz AM, Montag E, Arruda EG, et al. Superior-medial dermoglandular pedicle reduction mammaplasty for immediate conservative breast surgery reconstruction: technical aspects and outcome. Ann Plast Surg 2006;57:502-8. [PubMed]
- Clough KB, Nos C, Salmon RJ, et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg 1995;96:363-70. [PubMed]
- Nos C, Fitoussi A, Bourgeois D, et al. Conservative treatment of lower pole breast cancers by bilateral mammoplasty and radiotherapy. Eur J Surg Oncol 1998;24:508-14. [PubMed]
- Petit JY, Garusi C, Greuse M, et al. One hundred and eleven cases of breast conservation treatment with simultaneous reconstruction at the European Institute of Oncology (Milan). Tumori 2002;88:41-7. [PubMed]
- Losken A, Elwood ET, Styblo TM, et al. The role of reduction mammaplasty in reconstructing partial mastectomy defects. Plast Reconstr Surg 2002;109:968-75; discussion 976-7. [PubMed]
- Fitzal F, Nehrer G, Hoch D, et al. An oncoplastic procedure for central and medio-cranial breast cancer. Eur J Surg Oncol 2007;33:1158-63. [PubMed]
- Hudson DA. A modified excision for combined reduction mammoplasty and breast conservation therapy in the treatment of breast cancer. Aesthetic Plast Surg 2007;31:71-5. [PubMed]
- Munhoz AM, Montag E, Arruda EG, et al. The role of the lateral thoracodorsal fasciocutaneous flap in immediate conservative breast surgery reconstruction. Plast Reconstr Surg 2006;117:1699-710. [PubMed]
- Takeda M, Ishida T, Ohnuki K, et al. Breast conserving surgery with primary volume replacement using a lateral tissue flap. Breast Cancer 2005;12:16-20. [PubMed]
- Lee J, Bae Y, Audretsch W. Combination of two local flaps for large defects after breast conserving surgery. Breast 2012;21:194-8. [PubMed]
- McCulley SJ, Durani P, Macmillan RD. Therapeutic mammaplasty for centrally located breast tumors. Plast Reconstr Surg 2006;117:366-73. [PubMed]
- Chung TL, Schnaper L, Silverman RP, et al. A novel reconstructive technique following central lumpectomy. Plast Reconstr Surg 2006;118:23-7. [PubMed]
- Schoeller T, Huemer GM. Immediate reconstruction of the nipple/areola complex in oncoplastic surgery after central quadrantectomy. Ann Plast Surg 2006;57:611-5. [PubMed]
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006;117:1-11; discussion 12-4. [PubMed]
- Bajaj AK, Kon PS, Oberg KC, et al. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg 2004;114:1442-9. [PubMed]
- Munhoz AM, Montag E, Arruda EG, et al. Critical analysis of reduction mammaplasty techniques in combination with conservative breast surgery for early breast cancer treatment. Plast Reconstr Surg 2006;117:1091-103; discussion 1104-7. [PubMed]
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012;38:395-8. [PubMed]
- Bogusevicius A, Cepuliene D, Sepetauskiene E. The integrated evaluation of the results of oncoplastic surgery for locally advanced breast cancer. Breast J 2014;20:53-60. [PubMed]
- Staub G, Fitoussi A, Falcou MC, et al. Breast cancer surgery: use of mammaplasty. Results. Series of 298 cases. Ann Chir Plast Esthet 2008;53:124-34. [PubMed]
- Laucirica R. Intraoperative assessment of the breast: guidelines and potential pitfalls. Arch Pathol Lab Med 2005;129:1565-74. [PubMed]
- Kaur N, Petit JY, Rietjens M, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 2005;12:539-45. [PubMed]
- Giacalone PL, Roger P, Dubon O, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 2007;14:605-14. [PubMed]
- Rusby JE, Paramanathan N, Laws SA, et al. Immediate latissimus dorsi miniflap volume replacement for partial mastectomy: use of intra-operative frozen sections to confirm negative margins. Am J Surg 2008;196:512-8. [PubMed]
- Caruso F, Ferrara M, Castiglione G, et al. Therapeutic mammaplasties: full local control of breast cancer in one surgical stage with frozen section. Eur J Surg Oncol 2011;37:871-5. [PubMed]
- Brédart A, Petit JY. Partial mastectomy: a balance between oncology and aesthetics? Lancet Oncol 2005;6:130. [PubMed]
- Shestak KC, Johnson RR, Greco RJ, et al. Partial mastectomy and breast reduction as a valuable treatment option for patients with macromastia and carcinoma of the breast. Surg Gynecol Obstet 1993;177:54-6. [PubMed]
- Smith ML, Evans GR, Gürlek A, et al. Reduction mammaplasty: its role in breast conservation surgery for early-stage breast cancer. Ann Plast Surg 1998;41:234-9. [PubMed]
- Stolier A, Allen R, Linares L. Breast conservation therapy with concomitant breast reduction in large-breasted women. Breast J 2003;9:269-71. [PubMed]