Conservative mastectomies and immediate reconstruction with the use of ADMs
Introduction
In the last several decades, significant advancements have been made in the surgical management of breast cancer. Nipple-sparing mastectomies (NSM) and skin-sparing mastectomies (SSM) followed by immediate alloplastic breast reconstruction have emerged as oncologically safe treatment options yielding excellent cosmetic results. These techniques minimize breast deformity and optimize aesthetic outcome through preservation of the native skin envelope and restoration of a naturally looking breast mound using tissue similar in color, texture and sensation.
Traditionally, immediate implant-based breast reconstruction is performed in two stages using tissue expander/implant (TE/I) technique. Following mastectomy, the inferior border of the pectoralis major is released and a partially filled expander is placed in the submuscular pocket, often with inferior pole coverage provided by a thin serratus muscle/fascia flap. As such, sufficient coverage of the prosthesis is ensured and stress to the thin and vulnerable mastectomy skin flap is minimized. Post-operatively, serial expansions are followed by exchange of expander to implant once the desired breast size is achieved.
To eliminate delayed return to normal body image and minimize the burden of serial expansions and additional surgery associated with TE/I technique, a novel approach to immediate breast reconstruction has been introduced with the advent of acellular dermal matrix (ADM). ADM is an immunologically inert biomaterial prepared from xenoplastic or alloplastic cadaveric dermis devoid of cellular elements. It provides structurally intact tissue matrix that serves as a biological scaffold necessary for tissue ingrowth, angiogenesis and regeneration (1). In the setting of SSM or NSM where the entire native skin envelope is preserved, placement of ADM at the lower pole in continuity with the pectoralis major allows complete coverage of the prosthesis and provides additional support. The inferiorly placed ADM hammock suspends the prosthesis thus offloading mechanical stress from the overlying skin flap. Based on the quality of the skin envelope as well as surgeon preference, a decision can be made to either insert the permanent implant or alternatively insert a tissue expander.
Utilization of ADM confers several additional advantages including improved control over placement of the infra-mammary (IMF) and lateral mammary folds (LMF), preventing mechanical shift of the implant and stabilizing the pectoral muscle to minimize superior migration or window shading (2). Together, these can contribute to superior aesthetic outcomes of the reconstructed breast. Further, inferior placement of a dermal matrix may reduce rippling, visibility, palpability, bottoming-out and exposure of the implant (3,4). Reduced incidence of capsular contracture has also been reported (3).
The following manuscript presents the senior author’s 5-year experience with ADM in the setting of direct-to-implant breast reconstruction following SSM or NSM. A detailed description of surgical technique is provided along with a comprehensive discussion of patient selection and potential complications.
Materials and methods
Patients
A retrospective chart review of patients undergoing direct-to-implant breast reconstruction with the use of ADM (AlloDerm; LifeCell Corp., Branchburg, USA) was conducted at the Women’s College Hospital (Toronto, Ontario, Canada) over a 5-year period [2008-2013]. All operations were performed by the senior author using similar operative technique. Demographic data, previous radiation therapy and post-operative complications were recorded.
Candidates for direct to implant breast reconstruction are determined based on the indication for mastectomy, breast size and shape, BMI, patient co morbidities, patient preference as well as surgeon preference. Ideal candidates should be small breasted (A or B cup), with minimal ptosis and a nipple complex that requires minimal elevation on the breast mound. Generally direct to implant reconstruction is offered to women undergoing prophylactic mastectomy or mastectomy for pre-invasive disease. In some centers, patients with small invasive tumors are also offered single stage reconstruction. Patients should have a low or normal BMI (maximum BMI of 30), should be non-smokers and should not have undergone previous breast radiation.
Surgical technique
Prior to surgery, all patients receive a combination of medications that have been shown to assist in rapid recovery with minimal use of narcotics (5). This “cocktail” includes celebrex, acetaminophen and gabapentin. Intravenous antibiotics and a single dose of dexamethasone are administered in the operating room. Patients are positioned supine with arms abducted at 90 degrees.
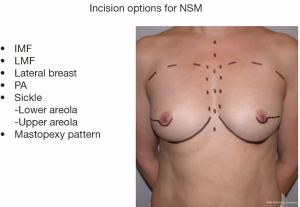
Various incisions have been described for both NSM and SSM (Figure 1). The choice of incision is based on the preference of the oncologic surgeon, the size of the breast and the location of existing pathology or previous scars. When performing a NSM, it is the author’s preference to use an IMF incision. In larger breasts, a mid breast incision extending from the areola may be used to allow easier access to the upper pole and the lateral breast tissue. It is important for the oncologic surgeon and the reconstructive surgeon to work collaboratively. As the mastectomy is started, the ADM is placed in a saline bath. The bath is changed every 15 minutes until the ADM is inserted. This assists with the removal of any preservatives that may be present from the processing of the material.
Following the mastectomy, the defect is carefully assessed. It is important to evaluate the quality of the skin envelope as well as the viability of the breast skin and the nipple areola complex. Assessment is performed clinically although various new technologies including the SPY Elite® System (LifeCell Corporation, Branchburg, USA) may be helpful in assessing tissue perfusion. The pectoral muscle as well as the serratus fascia is also assessed, as occasionally the muscle may be attenuated or damaged at the time of mastectomy. Prior to beginning muscle dissection, the pocket is irrigated vigorously to remove any loose tissue and fatty remnants. This is important to minimize infection and decrease the incidence of seroma formation.
Muscle elevation is performed with electro-cautery and begins along the IMF (Figure 2). This incision is carried laterally to include the serratus muscle fascia. Adding the fascia improves lateral implant stability and assists in defining the lateral mammary fold. The sub-pectoral pocket is dissected in a similar fashion to a breast augmentation, with pocket dimensions determined by the choice of a round or a shaped implant. The ADM is placed in the lower pole of the breast and oriented with the deep dermal side towards the breast skin. The ADM is secured to the inferior edge of the elevated muscle using interrupted and running absorbable suture. Several sutures are placed near the medial/inferior border. A sizer is inserted and several sutures are placed in the medial and lateral IMF. The bed is flexed to 90 degrees and the breasts are assessed for size, symmetry and fold position. The final implant is selected and the patient is returned to a supine position.
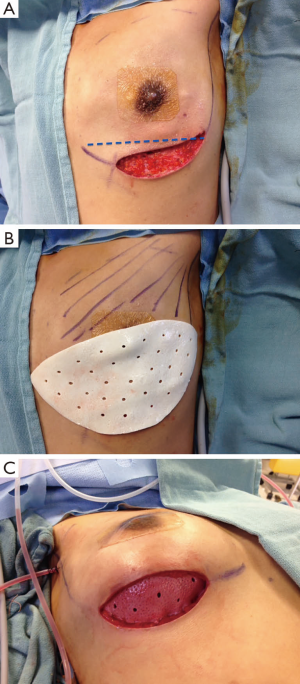
Two closed suction drains are inserted, one superficial and one deep to the ADM. Some surgeons chose to use one drain only, placed superficial to the ADM. The pocket is irrigated with antibiotic solution and the implant is inserted using a minimal or no touch technique. The ADM is then advanced inferiorly over the implant in order to secure a tight pocket for the implant. When a mid-breast incision is used, it is important to advance the ADM inferiorly to ensure that the pectoral muscle is sitting under the incision. The ADM is then secured to the IMF with a running absorbable suture.
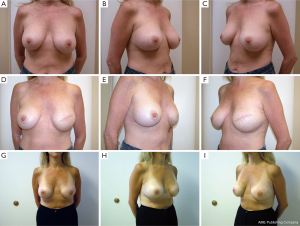
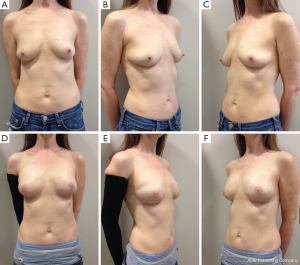
Following skin closure, the skin and the nipple areola complex are again checked for color and perfusion. A light dressing and a supportive sports bra are applied. Drains remain in place until they are draining less than 30 cc per day for 2 consecutive days. Patients are kept on antibiotics during this time period. Several case examples are shown in Figures 3-6.
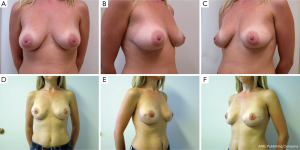
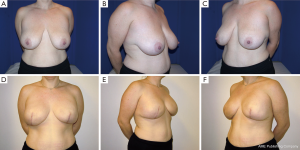
Results
Patient demographics
A total of 72 patients representing 119 breasts were identified. Mean patient age at time of surgery was 41.7 years (range, 28-62 years). Forty-seven patients underwent bilateral direct to implant reconstruction and 25 patients underwent unilateral direct to implant reconstruction. Among breasts operated, 45 (38%) cases were oncologic and 74 (62%) cases were prophylactic. Eighteen breasts (15.1%) undergoing reconstruction had a history of radiation to the reconstructed breast. Average follow-up was 16 months (range, 3-51 months).
There were approximately equal numbers of skin sparing and nipple sparing mastectomies (52% SSM, 48% NSM), however the percentage of nipple sparing mastectomies steadily increased during the period of study. All implants were silicone gel filled devices with the majority being shaped form stable implants (62% shaped 38% round).
Post-operative outcomes and complications
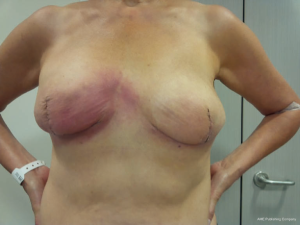
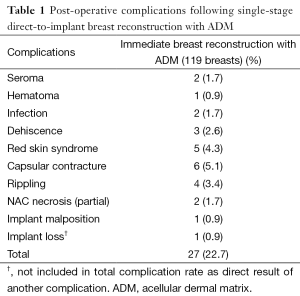
Overall, a total of 27 complications were recorded for a complication rate of 22.7% (27/119). Of the 119 breasts operated on, 116 successfully completed direct to implant reconstruction. One patient had an infection, which was treated with explantation and conversion to autogenous reconstruction. Two breasts with tissue necrosis or dehiscence had the implants removed and replaced with tissue expanders. These patients went on to successful reconstruction with an implant. Complications occurred in 23 out of the 72 patients (32%). The most common complication was capsular contracture (Baker III/IV), identified in six breasts. It should be noted that 4 of the 6 breasts with capsular contracture occurred in the 18 breasts that had undergone radiation therapy. Other complications included five cases of red skin syndrome, four cases of rippling, three cases of dehiscence and two cases of seroma (Figure 7). Most complications were treated non-surgically. Overall reoperation rate was 9.7% (7/72 patients). All red skin syndrome patients resolved with antibiotics and anti-inflammatories. Infection was recorded in two cases. Of these, one patient underwent removal of the implant and was subsequently treated with autologous reconstruction. The other case of infection was managed conservatively with oral antibiotics. Hematoma occurred in one patient. Partial NAC necrosis was noted in two breasts (1.7%). A list of complications appears in Table 1.
Full table
Discussion
Since the late 1990’s, a steady increase in implant-based breast reconstructions has caused a paradigm shift away from autologous tissue techniques. In 2008, alloplastic breast reconstruction comprised 68% of all reconstructive procedures performed in the United States (6). The 2-stage tissue expander to implant approach is the current gold standard for prosthetic breast reconstruction in North America. When compared to autologous reconstruction, it requires shorter operative times, eliminates donor site morbidity and allows for a more rapid convalescence (7,8). Notwithstanding, traditional implant-based breast reconstruction necessitates a series of visits for tissue expansion, a second surgical procedure and the eventual insertion of a permanent prosthesis, which will require ongoing maintenance and reoperations.
Since its introduction into reconstructive breast surgery by Breuing et al., ADM has gained acceptance as a safe and effective adjunct to surgery, permitting direct-to-implant reconstruction where the native skin envelope is preserved (9). In patients undergoing NSM or SSM, reconstruction with ADM provides internal support that stabilizes the implant position and minimizes pressure on the overlying skin flap. Placement of ADM at the lower pole in continuity with the sub-pectoral plane also confers the ability to control the inferior and lateral mammary folds, regardless of their potential violation during the mastectomy.
Additionally, supplementary coverage of the lower pole has been shown to decrease the incidence of rippling, bottoming out and implant migration when compared to non-ADM breast reconstructions (3). Other advantages of ADM-assisted direct-to-implant technique include reduced incidence of capsular contracture (Baker III/IV) and support of the pectoral muscle to minimize superior migration or window shading (3,10).
A wide assortment of alloplastic or xenoplastic dermal matrices have been used in breast reconstruction. Bovine-derived matrices include Tutomesh® (Novomedics GmbH, Bahnhofstrasse, Zürich) (11), Veritas® (Synovis, Minnesota, USA) (2) and SurgiMend® (TEI Biosciences, South Boston, USA) (12). Porcine-derived matrices include Strattice™ (LifeCell) (13,14) and Protexa® (Tencoss) (15). Finally, cadaveric ADM options include Flex HD® (Ethicon) (16), DermaMatrix® (Synthes) (17), NeoForm® (Mentor) (18) and AlloDerm® (Lifecell). The latter is commonly reported in the literature and represents the ADM used in this series.
The reported frequency of complications in direct to implant, ADM assisted breast reconstruction ranges from 3.9% (19) to 69.5% (20). Implant loss was reported from 0% to 17.4% of cases (21). With regard to specific major post-operative complications, seroma formation has the highest reported incidence, occurring in up to 17.8% of operated breasts (11). In our patient population, seroma was recorded in two breasts (1.7%). Multiple reports exist in the literature suggesting higher rates of seroma associated with the use of ADM. However, conflicting information exists. In a study of 415 immediate implant-based breast reconstructions performed with or without the use of ADM (269 ADM, 146 non-ADM), Chun et al. demonstrated a 4-fold increase in the rate of seroma formation (22). Conversely, in a study comparing 330 single-stage reconstructions with ADM to 148 two-stage TE/I reconstructions without ADM, Colwell et al. showed a low overall complication rate that was similar between both groups (14.8% for single-stage ADM vs. 19.6% for two-stage non-ADM, P=0.18). In their series, post-operative seroma was recorded in 1.5% vs. 1.9% of breasts reconstructed with or without ADM, respectively (P=0.81) (8). They suggested that in patients with a healthy skin envelope, ADM does not appear to increase the risk of complications and constitutes an important factor in the patient selection algorithm. Salzberg has also emphasized the importance of a healthy, well-vascularized and good quality skin flap in the clinical decision making process proceeding direct to implant breast reconstruction with ADM (4). Given contradictory evidence and lack of consensus, several technical precautions have been suggested to minimize the risk of seroma. These include placement of both sub-mastectomy and sub-ADM drains, decreased drain removal threshold (<20 cc/24 h) and post-operative use of a soft compression dressing and bra (23). It is also the author’s approach to vigorously irrigate the mastectomy pocket prior to ADM insertion to remove any residual fat from the pocket. Avascular fat has been shown to increase local inflammation and may predispose to a higher rate of seroma formation.
Infection leading to implant loss was the second most common major complication in our review of the literature; occurring in up to 13.0% of cases (21). Cellulitis managed conservatively was reported in up to 6.1% of breasts (20). Concern has been expressed in the literature regarding the “aseptic” and non-“sterile” nature of some ADMs available today and several studies suggest that these grafts are associated with higher infection rates. Chun et al. demonstrated a 5-fold increase in infection rate in ADM compared to non-ADM TE/I immediate breast reconstructions (269 ADM, 146 non-ADM) (22). Lanier et al. found a statistically significant higher rate of infection in the ADM group when comparing 75 ADM vs. 52 non-ADM TE/I breast reconstructions (28.9% vs. 12.0%, P=0.022) (24). Similarly, Liu et al. also demonstrated a statistically significant increase in overall wound infection rate in the ADM group compared with the non-ADM group in a cohort of 470 immediate TE/I reconstructions (6.8% vs. 2.5%, P=0.031) (16). However, as with seroma formation, literature is conflicting and numerous studies demonstrating no increased infection risk exist. A recent systematic review by Sbitany et al. comparing morbidity in ADM-assisted vs. non-ADM TE/I reconstruction illustrated similar rates of infection leading to explantation (3.2% for sub-muscular and 3.4% for ADM, P=0.18) and cellulitis/wound infection not requiring surgical intervention (2.8% vs. 3.4%, P=0.09) (25). In our series, a low overall infection rate was recorded (two cases) for a total infection rate of 1.7%. Of these, infection resulted in implant loss in one case (0.9%). In our institution, several preventative measures are employed to help minimize risk of infection during direct-to-implant reconstruction. The ADM is bathed 3 times for 10 minutes each in bacitracin and saline solution to remove any preservatives that may exist in the material. Further, utilization of new gloves for handling the ADM, copious irrigation of the ADM-pectoral pocket with bacitracin solution and minimal touch technique for manipulation of the final implant are used. Drains are inserted through separate incisions distant from the mastectomy incision and covered with sterile, waterproof dressings. Lastly, patients are continued on oral antibiotics until the drains are removed.
Mastectomy skin flap necrosis or skin breakdown requiring operative revision has been reported in up to 9.1% of cases (8). Minor skin flap necrosis or superficial epidermolysis managed conservatively was more frequent, reported in up to 28.7% of breasts (20). In our present study, wound dehiscence was recorded in three breasts (2.6%) and partial NAC necrosis in two others (1.7%). Several authors have suggested that larger pre-operative breast size and more significant breast ptosis are associated with higher likelihood of complications and failure in direct-to-implant reconstruction (20,26). Gdalevitch et al. demonstrated significantly higher mastectomy flap necrosis in D-cup breasts (OR, 6.25; P=0.027) and Roostaeian et al. showed higher revision rates in patients with D-cup breast size or greater (P=0.018) and grade two ptosis or greater (P=0.017) (20,26). As with any reconstructive procedure, patient selection is paramount and should include optimization of co-morbidities and identification of risk factors including large breast size, ptosis, smoking history, radiation as well as existence of previous breast scars.
In summary, ADM assisted direct-to-implant breast reconstruction has been shown to be a safe option for women who are candidates for nipple sparing or skin sparing mastectomies. The ability to preserve the breast envelope and restore volume with an implant that is supported in position by ADM can result in excellent aesthetic outcomes for the patient. It eliminates the need for frequent expansions and obviates the need for a planned secondary expander to implant exchange. This may assist in decreasing the physical and psychological impact of mastectomy and accelerate a return to normal life with a restored body image and improved quality of life. Judicious patient selection, careful evaluation of post-mastectomy skin flaps and consideration of possible risk factors for complications such as pre-operative breast size and ptosis are paramount to the success of this technique. Future studies including the ongoing Canadian Multi-Center Randomized Controlled Trial (MCCAT) will offer a rigorous comprehensive assessment of the direct to implant ADM-assisted approach and help to better define its future role in the field of reconstructive breast surgery (7).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:95S-103S. [PubMed]
- Mofid MM, Meininger MS, Lacey MS. Veritas® bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products. Eur J Plast Surg 2012;35:717-22. [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-10e. [PubMed]
- Salzberg CA. Direct-to-implant breast reconstruction. Clin Plast Surg 2012;39:119-26. [PubMed]
- Davidge KM, Brown M, Morgan P, et al. Processes of care in autogenous breast reconstruction with pedicled TRAM flaps: expediting postoperative discharge in an ambulatory setting. Plast Reconstr Surg 2013;132:339e-44e. [PubMed]
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [PubMed]
- Zhong T, Temple-Oberle C, Hofer S, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials 2013;14:356. [PubMed]
- Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg 2011;128:1170-8. [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [PubMed]
- Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75-89. [PubMed]
- Gubitosi A, Docimo G, Parmeggiani D, et al. Acellular bovine pericardium dermal matrix in immediate breast reconstruction after Skin Sparing Mastectomy. Int J Surg 2014;12:S205-8. [PubMed]
- Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg 2013;131:940-51. [PubMed]
- Lardi AM, Ho-Asjoe M, Mohanna PN, et al. Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg 2014;67:1098-105. [PubMed]
- Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg 2013;66:323-8. [PubMed]
- Potter S, Chambers A, Govindajulu S, et al. Early complications and implant loss in implant-based breast reconstruction with and without acellular dermal matrix (Tecnoss Protexa®): A comparative study. Eur J Surg Oncol 2015;41:113-9. [PubMed]
- Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg 2014;72:503-7. [PubMed]
- Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 2009;123:1-6; discussion 107-8. [PubMed]
- Losken A. Early Results Using Sterilized Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander Breast Reconstruction. Plast Reconstr Surg 2009. [Epub ahead of print]. [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [PubMed]
- Gdalevitch P, Ho A, Genoway K, et al. Direct-to-implant single-stage immediate breast reconstruction with acellular dermal matrix: predictors of failure. Plast Reconstr Surg 2014;133:738e-47e. [PubMed]
- Topol BM, Dalton EF, Ponn T, et al. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg 2008;61:494-9. [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [PubMed]
- Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg 2013;71:464-70. [PubMed]
- Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 2010;64:674-8. [PubMed]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [PubMed]
- Roostaeian J, Pavone L, Da Lio A, et al. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg 2011;127:1407-16. [PubMed]


