
This article has an erratum available at: http://dx.doi.org/10.21037/gs-2021-03 the article has been update on 2021-04-07 at here.
The expression of programmed death-ligand 1 in patients with invasive breast cancer
Introduction
Breast cancer is the most common type of cancer in women and it is the second leading cause of cancer death among women (1). While the incidence of breast cancer is higher in developed countries, less developed countries have higher relative mortality. Approximately 1.2 million women are diagnosed with breast cancer annually, and the incidence continues to rise at a rate of 5% to 20% a year (1). The pathogenesis of breast cancer is complex and involves alterations in signaling pathways and other changes at a molecular level. Risk factors for the disease include obesity (particularly after menopause), high-dose irradiation to the chest at a young age, dense breast tissue (or increased glandular tissue), the use of hormone replacement therapy, and family history (2). Symptoms of breast cancer include the presence of a painless firm mass, persistent changes in the breast (thickening, swelling, dimpling, distortion, tenderness, skin irritation, redness, scaling, and prominent superficial veins), and changes in the nipple such as ulceration, retraction or inversion, and spontaneous discharge (2).
The clinical treatment for breast cancer is largely dependent on the histological and molecular characteristics of the tumor. Depending on the stage of the cancer, treatment may include surgery, radiotherapy, chemotherapy, targeted therapy, or hormone therapy (3). In advanced breast cancer, the goal of treatment is to prolong life and control the symptoms by using treatments that have low toxicity, thereby improving quality-adjusted life expectancy. However, the clinical effectiveness of treatment is reduced by the high rates of recurrence, and metastasis associated with the disease. Moreover, there is currently a dearth of sensitive biomarkers for the early diagnosis of breast cancer (3).
Programmed death-ligand 1(PD-L1), also known as cluster of differentiation 274 or B7 homolog 1, is a 40 kDa type1transmembrane protein that has been speculated to play a key role in the suppression of adaptive immunity during pregnancy, tissue allografts, autoimmune diseases, and hepatitis (4). PD-L1 expression in tumors can serve as a criterion for selecting patients who will benefit from immunotherapy (4). Programmed death 1 (PD-1) receptor is found on the surface of activated T cells, and forms part of the immune checkpoint that prevents the destruction of healthy host cells. Tumor cells can express surface PD-L1 or PD-L2. Binding of PD-L1 or PD-L2 to the PD-1 receptor inhibits T-cell activation (TCR) and leads to T cell apoptosis and the inhibition of cytokine production (5,6). Hence, PD-1 and its ligand, PD-L1, are key physiological suppressors of the cytotoxic immune reaction. Therefore, tumors with increased expression levels of PD-1 will likely result in poor prognosis. In fact, studies have reported levels of PD-L1 expression to be negatively correlated with the prognosis of patients and the degree of tumor malignancy (7). The activity of specific antitumor T cells was restored via checkpoint blockade using anti-PD-1 and anti-PD-L1 antibodies (7). Furthermore, the expression of PD-L1 has been shown to be altered in many solid tumors, such as lung, gastric, and colorectal cancers (6,8). However, little is known about the role of PD-L1 expression in the pathogenesis of breast cancer. This study aimed to investigate the associations between PD-L1 protein expression and the clinicopathological features of patients with invasive breast cancer.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/gs-20-824).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Affiliated Taikai Xianlin Drum Tower Hospital (NO. 2018-02) and informed consent was taken from all individual participants.
Patients and general information
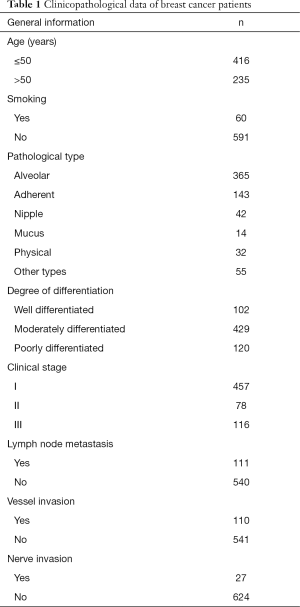
A total of 651 female patients with invasive breast carcinoma were recruited over a from June 2019 to June 2020. The clinicopathological data of the patients are shown in Table 1.
Full table
Assay of hormone receptor status and classification of patients
Breast biopsies were taken from each patient and the expression of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) protein was assessed. Patients whose tissue samples did not express genes for these receptors were classified as
Triple Negative Breast Cancer (TNBC). Preoperative puncture biopsy was performed in this group of patients: (I) the clinician used an adjustable automatic biopsy gun to perform 4 rounds of biopsy on different areas of the patient’s breast mass. (II) The specimens were immobilized with 10% neutral formalin solution and embedded with paraffin. (III) Paraffin sections with a thickness of 5 µm were prepared, stained with HE, and observed under an optical microscope.
Immunohistochemical staining
To measure the levels of PD-L1 expression, biopsies were taken from the patients, and the samples were processed for immunohistochemical staining. Tissue sections (3 µm thick) were deparaffinized, rehydrated, and incubated in 3% H2O2 for 10 minutes to reduce non-specific background staining. The tissue sections were then incubated in 10 mM citrate buffer (pH 6.0) for 15 minutes in a microwave oven, with agitation. This was followed by incubation with an Ultra V Block solution (Sigma, Shanghai, China) for 10 minutes at room temperature. A rabbit anti-PD-L1 monoclonal antibody (1:250) (Abcon Trading Co., LTD., Shanghai, China) was added, and the tissue sections were incubated for 2 hours at room temperature. Antibody binding was determined using the Ultra-vision LP System according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). The sections were developed using 3,3’ Diaminobenzidine tetrahydrochloride (DAB), and counterstained with hematoxylin. The levels of PD-L1 expression were assessed using to the Allred scoring system. A score of 0 was considered to represent negative PD-L1 expression, while scores of 1+ to 4+ were interpreted as over-expression of PD-L1.
Correlation analysis
The relationships between the levels of PD-L1 expression and the clinicopathological characteristics of patients were determined using Pearson’s correlation coefficient.
Univariate and multivariate analyses
Logistic binary regression analyses were used to predict the risk factors affecting PD-L1 expression in breast cancer patients.
Statistical analyses
Statistical analyses were performed using the SPSS software (Version 20.0). Groups were compared using Chi-square tests. Results were considered statistically significant when P<0.05.
Results
Expression levels of PD-L1 expression in breast cancer tissues
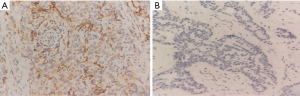
PD-L1 expression was observed in tumor-infiltrating lymphocytes in the breast cancer tissue samples, and its expression was localized in the cytoplasm and cell membrane, presenting as diffuse brownish yellow granules (Figure 1A). It was not expressed in healthy human lymphocytes (Figure 1B). Positive expression of PD-L1 was detected in 47% of patients with invasive breast carcinoma, compared to 69.3% of TNBC patients (P<0.05; Tables 2,3).
Full table

Full table
Correlations between PD-L1 expression and different clinicopathological characteristics of patients with invasive breast cancer
The expression of PD-L1 in patients with invasive breast cancer was significantly correlated with WHO grade, tumor size, vascular invasion, pathological stage, and positive expression of ER, PR, Ki67, p53, cytokeratin 5/6 (CK5/6), and epidermal growth factor receptor (EGFR) (P<0.05; Table 2).
Correlation of PD-L1 expression with different clinicopathological characteristics in patients with TNBC
In TNBC patients, the expression of PD-L1 was significantly correlated with WHO grade, neuro-invasion, and expression of Ki67, CK5/6, and EGFR (P<0.05, Table 3). However, it was not correlated with age, tumor size, vascular invasion, number of lymph nodes, pathological stage, or expression of ER, PR, p53, androgen receptor (AR), or VEGFR (P>0.05, Table 3).
Results of univariate analysis and multivariate analysis
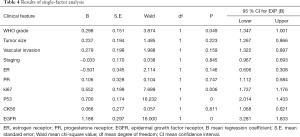
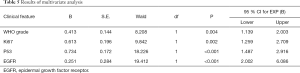
Logistic binary regression single-factor and multivariate analysis revealed WHO grade, and Ki67, p53, and EGFR expression to be risk factors for PD-L1-expressing breast carcinoma (Tables 4,5).
Full table
Full table
Discussion
Breast cancer is a malignant tumor that originates from the epithelial cells of the terminal unit of the breast. Accounting for 10.4% of all cancers among women worldwide, it is the second most common non-skin cancer after lung cancer. Breast cancer ranks second as a cause of female tumor-related mortality (9). Although breast cancer is treatable if diagnosed early, the efficacy of current treatment strategies is generally unsatisfactory due to widespread chemo-resistance and the absence of any effective indicators that can predict and monitor disease progression. The high rate of metastasis associated with breast cancer contributes to poor prognosis and reduces the overall survival of patients (10). Metastatic or stage IV breast cancer is the most advanced type of breast cancer. In most stage IV cases, breast cancer spreads to nearby lymph nodes and further through the body to areas such as the bones, lungs, liver, and the brain (10). Annually, an estimated 1 million cases of breast cancer are diagnosed globally, with more than 170,000 cases classified as triple-negative breast cancer (TNBC) (11). TNBC is characterized by the negative expression of the ER and the PR, and an absence of HER2 protein overexpression. It is a subtype of breast cancer that overlaps with "basal-like" breast cancer (11). As there is currently no effective targeted therapy for TNBC, patients with this subtype tend to have a poor prognosis. At present, TNBC has been proved to be an immunogenic tumor, and the use of systemic immunotherapy enables the autoimmune system to directly destroy targeted tumor cells, which has been used as an effective treatment for TNBC (12).
Programmed death 1 (PD-1) is a receptor expressed on the surface of T cells, B cells, and natural killer (NK) cells, where it regulates their activation and apoptosis. Its ligand, PD-L1, is expressed in some tumor cells, activated B and T cells, dendritic cells, macrophages, and fibroblasts (13). PD-L1, as a transmembrane protein immunosuppressive molecule of PD-1 ligand, can inhibit the activation process of T lymphocytes when combined with PD-1. Blockade of the PD-1/PD-L1 pathway with monoclonal antibodies (against PD-1 or PD-L1) is a promising therapeutic approach that is currently being trialed in various types of human cancers (14,15). The expression of PD-L1 has been shown to be altered in numerous solid tumors, such as breast cancer, lung cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, testicular cancer, and papillary thyroid cancer. Moreover, several meta-analyses have demonstrated that its overexpression signifies poor prognosis in many cancers (16). However, little is known about the expression of PD-L1 in breast cancer, and its prognostic significance remains unclear. A study by Costa et al. reported that 45% of TNBC patients showed positive PD-L1 expression (17). The expression was positively correlated with tumor size, degree of differentiation, Ki-67 proliferation, negative ER and PR expression, and positive HER2 protein expression. However, PD-L1 was found to be negatively correlated with survival (18). In other studies, 20–25% of TNBC patients showed positive PD-L1expression (19,20). It has been reported that the levels of PD-L1 expression may be an important risk factor for breast cancer (21).
The results of this study showed that the positive expression rate of PD-L1 in invasive breast cancer was 47.0%, and the positive expression rate of TNBC, the special subtype, could also reach 69.3%, which was close to the above results. PD-L1 expression in patients with invasive breast cancer was significantly different in different WTO grades, tumor size, vascular invasion, pathological stage, ER, PR, Ki67, P53, CK5/6 and EGFR (P<0.05); In TNBC patients, the expression of PD-L1 was significantly correlated with WHO grade, nerve invasion, Ki67, CK5/6 and EGFR (P<0.05). These results suggest that the expression of PD-L1 is closely related to most pathological features of patients with invasive breast cancer, which may reflect tumor burden. Further univariate and multivariate analysis showed that the expression of PD-L1 was significantly correlated with WHO staging, Ki67, P53 and EGFR. WHO staging, Ki67, P53 and EGFR were independent risk factors affecting the expression of PD-L1, and the expression level of PD-L1 might be related to these factors.
Conclusions
More and more evidence shows that the activation of PD-1/PD-L1 signaling pathway can restore T lymphocytes' control over tumor cells, making tumor cells unable to escape immune, thus killing tumor cells. This study confirmed that the PD-L1 in breast ductal carcinoma especially in the patients with TNBC high expression, and PD-L1 and some clinical pathological features between the complex and the close relation. This means that immunotherapy with PD-L1 inhibitors can be used as a potential treatment strategy for patients with invasive breast cancer with high expression of PD-L1, and providing theoretical basis for further research and clinical promotion.
Acknowledgments
Funding: Project of Nanjing Health Committee (YKK19176).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-824
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-824
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-824). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Affiliated Taikai Xianlin Drum Tower Hospital (NO.2018-02) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun YS, Zhao Z, Yang ZN, et al. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci 2017;13:1387-97. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288-300. [Crossref] [PubMed]
- Hansen JD, Du Pasquier L, Lefranc MP, et al. The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol 2009;46:457-72. [Crossref] [PubMed]
- Lu Q, Qin T, Xu F, et al. Clinical implication of platelet-lymphocyte ratio and PD-L1 in breast cancer patients. Transl Cancer Res 2018;7:659-67. [Crossref]
- Ameratunga M, Asadi K, Lin X, et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS One 2016;11:e0153954. [Crossref] [PubMed]
- Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24. [Crossref] [PubMed]
- Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J 2014;20:256-61. [Crossref] [PubMed]
- Kolak A, Kamińska M, Sygit K, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med 2017;24:549-53. [Crossref] [PubMed]
- Aksoy S, Dizdar O, Harputluoglu H, et al. Demographic, clinical, and pathological characteristics of Turkish triple-negative breast cancer patients: single center experience. Ann Oncol 2007;18:1904-6. [Crossref] [PubMed]
- Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018;124:2086-103. [Crossref] [PubMed]
- Yu LY, Tang J, Zhang CM, et al. New Immunotherapy Strategies in Breast Cancer. Int J Environ Res Public Health 2017;14:68. [Crossref] [PubMed]
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674-90. [Crossref] [PubMed]
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361-70. [Crossref] [PubMed]
- Thompson E, Taube JM, Elwood H, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 2016;29:249-58. [Crossref] [PubMed]
- Chawla A, Philips AV, Alatrash G, et al. Immune checkpoints: A therapeutic target in triple negative breast cancer. Oncoimmunology 2014;3:e28325. [Crossref] [PubMed]
- Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat 2018;169:397-406. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:324-54. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Emena LA, Braiteh FS, Gassier P. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res 2015;75:abstr 2859.
- Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006;8:190-8. [Crossref] [PubMed]
(English Language Editors: J. Teoh and J. Reynolds)


