
The clinical characteristics and survival associations of pancreatic neuroendocrine tumors: does age matter?
Introduction
Pancreatic neuroendocrine tumor (pNET) is the second most common pancreatic neoplasm with relatively inert biological behavior compared to pancreatic adenocarcinoma (PDAC) (1). However, its incidence has increased steadily in recent decades (2), most likely due to more sensitive detection methods and more frequent routine examinations (3,4). Recent reports suggest that early-onset pancreatic cancer (EOPC), defined as PDAC with onset age before 50, shows a different clinical characteristics pattern and overall survival rate compared with other patients with this disease. EOPC constitutes 5–10% of all PDAC cases (5-7). According to previous reports, the mean age at diagnosis of pNET is 56.8 years, with a small proportion of cases diagnosed at a younger age (8). However, there has been little research on the differences between early-onset pNETs (EOpNETs) and typical age-at-onset pNETs (TOpNETs), probably due to the difficulty in studying a rare disease subset with lower incidence. Most studies are descriptive and only include relatively small populations lacking a typical age-at-diagnosis comparison group. Wang et al. reported that the onset age significantly impacts overall survival and should be considered in future pNETs staging systems (9). To identify whether the onset age of pNET shows similar differences to those seen in PDAC, we conducted this study to evaluate the associations between clinical characteristics and prognosis by age at diagnosis in pNET, and whether EOpNET differs from TOpNET. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-634).
Methods
Patients and data collection
Pathologically confirmed pNET cases from 2004–2015 were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database. Data from patients with pancreatic cancer were collected according to the 2nd and 3rd editions of the International Taxonomy (ICD-O-2/3): C25.0 to C25.9.9. The diagnostic codes used for searching are as follows: 8150 (pancreatic endocrine tumor), 8151 (insulinoma), 8152 (glucagonoma), 8153 (gastrinoma), 8155 (vipoma), 8156 (somatostatinoma), 8240 (carcinoid tumor), 8241 (enterochromaffin cell carcinoid), 8242 (enterochromaffin-like cell tumor), 8243 (goblet cell carcinoid), 8246 (neuroendocrine carcinoma), and 8249 (atypical carcinoid tumor). Tumor, nodes, and metastasis (TNM) data were collected according to the codes below: derived from the American Joint Committee on Cancer (AJCC) stage group 6th ed. (2004+), collaborative stage (cs) tumor size 2004, lymph nodes 2004, metastasis at dx 2004, regional nodes positive (1988+), and regional nodes examined (1988+). Basic clinicopathologic characteristics were retrieved, including gender, age, race, surgery, location of the primary site, and differentiation.
Subsequently, a single-center series of the Fudan University Shanghai Cancer Center (FUSCC) was analyzed, and patients with a pathological diagnosis of pNET were included. The demographic data, including gender, age, grade, location of the primary site, and surgical status, were collected. Data related to tumor T stage, nodal status, and metastases were also retrieved and classified according to the 8th AJCC staging classification. The follow-up data were confirmed by monthly review of medical records and by contacting patients or their relatives to ascertain disease progression or death date if applicable.
Our hospital Ethics Committee approved the study (050432-4-1805C), and written informed consent was received from all patients. We excluded patients in both cohorts who had an overall survival time of fewer than 3 months to rule out perioperative mortality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
For statistical analysis, we defined patients aged <50 years at the time of diagnosis as EOpNET and those aged ≥50 years as TOpNET Chi-squared tests or sample t-tests compared means for baseline clinical features. The survival time was defined from the date of the first diagnosis to the last follow-up or death. Log-rank tests were used to analyze the overall survival, and the Kaplan-Meier method was used to compare the survival proportions. Univariate survival analysis of variables such as age, sex, grade, surgery, and tumor location was performed by Cox proportional hazards regression. and hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated. SPSS Statistics version 21.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 was used for the statistical analyses. All tests were two-sided, and tests with P values <0.05 were considered statistically significant.
Results
In total, 5,368 patients were pathologically diagnosed with pNET in the SEER database and included in the study (Table 1), including 1,203 (22.4%) EOpNET patients {age range [15–19, 50]} and 4,165 (77.6%) TOpNET patients {age range [50, 80+]}, respectively. The male/female ratio was around 1:1 in EOpNET patients (1:1.05), similar to previous reports (7,8). The proportion of males in the TOpNET group was significantly higher than females (1.30:1, P<0.001). Approximately half of the patients had a tumor located in the body and/or tail of the pancreas in both groups (45.2% and 48.1%). 92.5% and 89.4% of patients had low or intermediate differentiated tumors in the EOpNET and TOpNET groups, respectively. Furthermore, a significantly higher proportion of patients in the EOpNET group received surgery (P<0.001) than in the TOpNET group. The median survival period of the EOpNET group was 136 [3–143] months compared to 85 [3–143] months (P<0.001) for the TOpNET group. Factors associated with survival were evaluated by Univariate Cox proportional hazards models in both groups. We found that even though gender did not affect the EOpNET group’s survival, females had a lower risk of death than males in the TOpNET group (HR: 0.871, 95% CI: 0.79–0.97, P=0.010). Moreover, married individuals had a lower risk of death than single people in both the EOpNET (HR: 0.729, 95% CI: 0.57–0.94, P=0.014) and TOpNET groups (HR: 0.843, 95% CI: 0.71–1.00, P=0.047). Compared to patients diagnosed between 2004–2009, patients diagnosed between 2010–2015 had significantly better prognoses in both the EOpNET (HR: 0.750, 95% CI: 0.59–0.96, P=0.020) and TOpNET groups (HR: 0.799, 95% CI: 0.71–0.89, P<0.001). Concerning the T stage of pNET, we found that T2 stage patients had a 3.167-fold increased death risk compared to T1 stage patients in the TOpNET group (HR: 3.167, 95% CI: 2.40–4.19, P<0.0001), while there were no differences in the EOpNET group (HR: 1.294, 95% CI: 0.80–2.11, P=0.300). Similar results were observed in patients with a pathological grade II differentiation. Compared to tumors located in the pancreatic head, tumors in the body (HR: 0.712, 95% CI: 0.60–0.85, P=0.001) and tail (HR: 0.721, 95% CI: 0.63–0.83, P<0.001) had a lower risk of death in the TOpNET group, while the location had no effect on survival in the EOpNET group. Surgery reduced the risk of death in both EOpNET (HR: 0.128, 95% CI: 0.10–0.16, P<0.001) and TOpNET groups (HR: 0.191, 95% CI: 0.17–0.22, P<0.001), respectively.
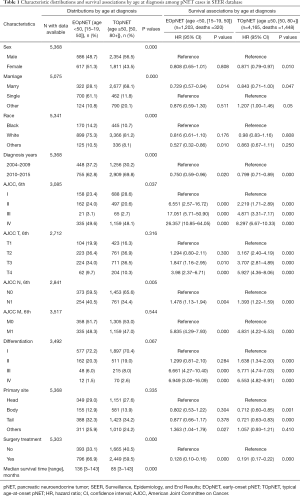
Full table
Also, a total of 330 patients from the FUSCC were pathologically diagnosed with pNETs and included in this study (Table 2). They comprised 126 (38.2%) EOpNET {age range [16, 50]} and 204 (61.8%) TOpNET patients {age range [50, 81]}, respectively. The proportion of females in the EOpNET group was higher than in the TOpNET group, but this difference was not statistically significant (P=0.362). The ratio of tumor location (head: body and/or tail) in both groups’ pancreas was 1:1.7. The majority of patients had G1 or G2 tumors in both the EOpNET (87.0%) and TOpNET groups (86.5%). Compared to the SEER database, a higher proportion of our institution patients received surgery in both the EOpNET and TOpNET groups, but this difference was not statistically significant (P=0.977). The median follow-up time in the EOpNET group was 47.97 (3.6–152.83) and 43.87 (4.33–128.67) months in the TOpNET group. G2 and G3 tumors had a 9- and 30-fold increased risk of death, respectively, compared to G1 tumours in the EOpNET group (HR: 9.437, 95% CI: 1.22–73.16, P=0.032; HR: 30.44, 95% CI: 3.81–243.50, P=0.001). However, the risk of death in the TOpNET group were only 1.468- and 4.614-fold greater, respectively (HR: 1.468, 95%CI: 0.70–3.07, P=0.308; HR: 4.614, 95% CI: 1.98–10.74, P<0.001). This shows that the differentiation in the grade of the tumor plays an important role in the progression of EOpNET patients. Furthermore, following the results in the SEER cohort, surgery reduced the risk of death significantly in both the EOpNET (HR: 0.080, 95% CI: 0.03–0.20, P<0.001) and TOpNET groups (HR: 0.168, 95% CI: 0.09–0.31, P<0.001). In addition, more patients received surgical resections with curative intent rather than other palliative/cytoreductive surgery in the EOpNET group (95.3% vs. 4.7%) compared to the TOpNET group (85.0% vs. 15.0%) (P=0.007).
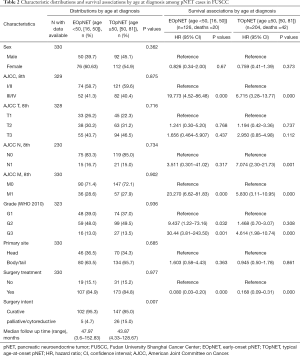
Full table
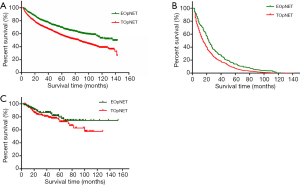
We further analyzed the overall survival associations by age at diagnosis of pNET in the two cohorts using the Kaplan Meier method, and survival curves were formulated (Figure 1). We found that in the SEER cohort, the overall survival of EOpNET patients was significantly better than that of TOpNET patients {136 [3–143] vs. 85 [3–143] months, P<0.001} with a median follow-up time of 92 [3–143] months. To exclude other causes of death in pNET patients, we conducted analyses according to the specific cause of death, and the outcome was similar {23 [3–142] vs. 16 [3–142] months, P<0.001}, with a median follow-up time of 18 [3–142] months. In the FUSCC cohort, the overall survival in EOpNET patients was also better than TOpNET patients with a median follow-up time of 39.585 (3.6–152.8) months (n=330), but this was not statistically significant (P=0.245), most likely due to the relatively small sample size.
Discussion
pNETs are relatively rare tumors. Their incidence is approximately 5.25/100,000, and the majority have a favorable prognosis (4). However, their clinical features and prognosis are highly heterogeneous, and among them, a small percentage of patients display malignant characteristics. For example, the overall 5-year survival in metastatic non-functional pNET is only 30%, presenting challenges for clinical practice (10). Therefore, clarifying the clinical characteristics and survival associations could be of great value in managing pNETs.
In our study, the proportion of EOpNET in the FUSCC cohort (38.2%) was significantly higher than in the SEER cohort (28.9%). The average age at diagnosis of pNET in our cohort was 52.6±12.6 years, which is younger than the SEER cohort 62±15 years (4). The mean age of diagnosis of PDAC in the USA is reportedly 71 years, whereas, in China, it is 62–65 years (11,12). Hence, it is reasonable to examine the variations among different subgroups and ethnicities, and the similarities in conditions in PDAC. The overall survival of EOpNET {136 [3–143] months} was significantly better than TOpNET {85 [3–143] months} (P<0.001) in the SEER database, but although this was also the case in the FUSCC cohort, who had a median follow-up time of 39.585 (3.6–152.8) months (n=330), the FUSCC cohort difference was not statistically significant (P=0.245), which is consistent with the previous reports (9). In this study, we analyzed both cohorts at 50 years of age for consistency; however this could be the reason that certain characteristics in the FUSCC cohort are not consistent with, or as obvious as, the SEER database. Therefore, exploring the reasons for the different ages of disease onset in different states and ethnicities could provide new strategies for future therapy.
Surgery is the most effective therapy for localized tumors, and in metastatic NETs, where more than 90% of liver tumors can be resected, cytoreductive surgery is recommended (2). On the other hand, according to Haynes et al., even small tumors can be aggressive and may require resection (13). Our study indicates that EOpNETs have a better prognosis than TOpNETs; therefore, it seems that as soon as a patient receives a diagnosis of pNET, surgery should be considered a priority in order to maximize survival rate. Even small tumors that need follow-up can cause significant discomfort and stress for patients and impact their quality of life on multiple levels. Therefore, we suggest that earlier resection is warranted for all patients with pNET. In our study, we also found that surgery can significantly lower the risk of death in both the EOpNET and TOpNET groups. Furthermore, tumors located in the body and tail posed a lower risk of death than those located in the pancreatic head in TOpNET patients, but not in EOpNET patients. This may be attributed to the poorer physical condition of older patients in the TOpNET group, or that patients with tumors located in the pancreatic head underwent relatively more invasive surgery of pancreaticoduodenectomy.
We also found that patients diagnosed between 2010–2015 had a significantly better prognosis than those diagnosed between 2004–2009, suggesting that earlier tumor detection is occurring in recent years due to more prevalent routine medical examinations and the adoption of systemic treatment options for pNET. Somatostatin analogs, peptide receptor radionuclide, and everolimus have been demonstrated to improve the prognosis of pNET effectively. Combined chemotherapy with temozolomide and capecitabine has also recently been found to prolong the progression-free survival of pNETs (14,15). And indeed, a substantial proportion of patients in both cohorts in our study did not undergo surgery but rather received other treatments (somatostatin analogs, chemotherapy, etc.). This may have impacted the survival analysis of our data but unfortunately could not be adjusted because of the incomplete patient information and nonstandardized treatment protocols. Interestingly, our study also shows that single patients had a significantly higher risk of death than married patients, which agrees with previous reports suggesting that social support could potentially and significantly impact cancer survival rates (16).
There is no consensus yet on the optimal follow-up strategy for pNET. Most guidelines suggest performing enhanced CT or magnetic resonance imaging (MRI) of the abdomen yearly for the first 3 years, then every 1 to 2 years for a total of 10 years (17). For tumors smaller than 2 cm, previous reports lack agreement about the need for observation or surgery. From the perspective of this research, we would recommend surgery as the first line of treatment given that the EOpNET group had a significantly better overall survival than the TOpNET group. It would seem that the earlier a patient receives surgery, the better their prognosis.
pNETs are usually sporadic, but increasing evidence points to the important role genetic mutations play in initiating and developing pNETs. Jiao et al. performed whole-exome sequencing in 68 sporadic pNETs and found that MEN1 (multiple endocrine neoplasia types 1) and DAXX/ATRX (death domain-associated protein/α-thalassemia mental retardation syndrome) mutations exist in more than 40% of pNETs. Also, approximately 14% of specimens had mutations in the mTOR pathway genes, which indicates that these may be potential therapeutic targets (18). Scarpa et al. performed whole-genome sequencing in 102 primary pNETs and found that germline mutations play a greater than expected role in clinically sporadic pNETs (3). Our team is also working on the multi-omics studies of pNET to clarify the pathogenesis to find novel therapeutic targets that can improve prognosis. According to the “two-hit” hypothesis (19), gene mutations in tumors are closely associated with the onset age. Therefore, future studies on the variations in genetic mutations among different age groups, especially in early-onset tumors, could help identify pNET driver genes.
In this current retrospective study, all patients were histology diagnosed; hence they were either recruited from a surgical database or positive biopsy results; thus, there may be some bias in the proportion of different stages. However, we screened the SEER database and our institution to minimize the influence of inadequate sample size and geography. Thus, the clinical characteristics of EOpNET and TOpNET and their effects on prognosis were supported and complemented by both cohorts. However, when exploring the survival associations with age at diagnosis, our data was not statistically significant, and this may be due to the limited number of samples and the number of deaths. Young people are more likely to be diagnosed with functioning pNETs and syndromic-related pNETs (i.e., MEN-1 or VHL), which seem to have a different biological profile sporadic pNETs (20,21), and this may have affected the results of the EOpNET group to some extent.
On the other hand, the proportion of elderly and very elderly patients in the TOpNET group may also have affected our overall survival results, as Li et al. noted that elderly patients have a poorer physical condition and are less likely to undergo surgery (22). Another limitation of this study is that we did not investigate the type of tumor function. Although only a small percentage of pNETs are known to be functional, this may have caused some bias in prognosis, because functioning neoplasms usually have a better prognosis than nonfunctioning tumors as a result of early diagnosis.
EOpNET constitutes only 28% to 38% of pNET patients, and these individuals tend to have a better prognosis. However, due to the younger age of onset, the disease is responsible for a greater proportion of years-of-life-lost. Therefore, identifying the clinical characteristics and prognostic associations by the onset age of pNET is important in gaining a better understanding of EOpNET, and may potentially yield strategies to either delay the onset of disease or to assist in formulating new therapeutic methods.
Conclusions
The EOpNET group demonstrated a significantly better overall survival time than the TOpNET group, and for this reason, early surgical resection is encouraged for all pNET patients. Identifying the clinical characteristics and prognostic associations by the age of onset in pNET can help our understanding of pNET, potentially yield strategies to delay the onset of disease, or assist in promoting new therapeutic methods. In any future personalized treatment of pNET, patients’ onset age will become another important factor for guiding treatment and prognosis.
Acknowledgments
We thank Professor Min Li, for reviewing our manuscript for clarity.
Funding: This work was supported by the National Science Fund for Distinguished Young Scholars [grant number 81625016]; The Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057); The National Natural Science Foundation [grant numbers 81502031, 81372651 and 81972725]; The Shanghai Municipal Science and Technology Commission (19QA1402100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-634
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-634
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-634
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-634). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee approved the study of Fudan University Shanghai Cancer Center (050432-4-1805C). All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer, 2010.
- Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin68:471-87.
- Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017;543:65-71. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Ben-Aharon I, Elkabets M, Pelossof R, et al. Genomic landscape of pancreatic adenocarcinoma in younger versus older patients: does age matter? Clin Cancer Res 2019;25:2185-93. [Crossref] [PubMed]
- McWilliams RR, Maisonneuve P, Bamlet WR, et al. Risk factors for early-onset and very-early-onset pancreatic adenocarcinoma: a pancreatic cancer case-control consortium (PanC4) analysis. Pancreas 2016;45:311-6. [Crossref] [PubMed]
- Beeghly-Fadiel A, Luu HN, Du L, et al. Early onset pancreatic malignancies: clinical characteristics and survival associations. Int J Cancer 2016;139:2169-77. [Crossref] [PubMed]
- Luo G, Javed A, Strosberg JR, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J Clin Oncol 2017;35:274-80. [Crossref] [PubMed]
- Wang Z, Jiang W, Zheng L, et al. Consideration of age is necessary for increasing the accuracy of the AJCC TNM staging system of pancreatic neuroendocrine tumors. Front Oncol 2019;9:906. [Crossref] [PubMed]
- Jilesen AP, van Eijck CH. Postoperative complications, in-hospital mortality and 5-year survival after surgical resection for patients with a pancreatic neuroendocrine tumor: a systematic review. World J Surg 2016;40:729-48. [Crossref] [PubMed]
- Jia X, Du P, Wu K, et al. Pancreatic cancer mortality in China: characteristics and prediction. Pancreas 2018;47:233-7. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011;146:534-8. [Crossref] [PubMed]
- de Mestier L, Walter T, Evrard C, et al. Temozolomide alone or combined to capecitabine for the treatment of advanced pancreatic NET. Neuroendocrinology 2020;110:83-91. [Crossref] [PubMed]
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [Crossref] [PubMed]
- Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31:3869-76. [Crossref] [PubMed]
- Singh S, Moody L, Chan DL, et al. Follow-up recommendations for completely resected gastroenteropancreatic neuroendocrine tumors. JAMA Oncol 2018;4:1597-604. [Crossref] [PubMed]
- Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. [Crossref] [PubMed]
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 1971;68:820-3. [Crossref] [PubMed]
- Sadowski SM, Triponez F. Management of pancreatic neuroendocrine tumors in patients with MEN 1. Gland Surg 2015;4:63-8. [PubMed]
- Oleinikov K, Uri I, Jacob H, et al. Long-term outcomes in MEN-1 patients with pancreatic neuroendocrine neoplasms: an Israeli specialist center experience. Endocrine 2020;68:222-9. [Crossref] [PubMed]
- Li G, Tian ML, Bing YT, et al. Clinicopathological features and prognosis factors for survival in elderly patients with pancreatic neuroendocrine tumor: a STROBE-compliant article. Medicine (Baltimore) 2019;98:e14576. [Crossref] [PubMed]


