
Concomitant use of 18F-FDG PET-CT SUVmax is useful in the assessment of Ki67 labeling index in core-needle biopsy specimens of breast cancer
Introduction
Ki67 is a nuclear protein that is expressed in the G1, S, G2, and M phases of the cell cycle, but not in resting cells in G0 phase. It has been used as an index of tumor proliferation (1). A large number of studies have demonstrated the prognostic value of the Ki67 labeling index (Ki67LI) (2-5). On the other hand, Ki67LI shows a different significance in predicting response to neoadjuvant chemotherapy (6-8). For example, although low-Ki67LI tumors generally do not show a favorable response to chemotherapy, they exhibit good prognosis. On the other hand, high-Ki67LI tumors are generally sensitive to chemotherapy, and a high Ki67LI is associated with increased chances of pathological complete response (7) and improved survival (6,8). However, some of the latter tumors display a highly aggressive phenotype, high resistance to conventional chemotherapy, and poor survival. Thus, although the predictive significance of Ki67LI remains undetermined, Ki67LI evaluation is largely employed in clinical practice in the selection of the therapeutic strategy. However, “no ideal Ki67LI threshold has been defined yet (9). St. Gallen international expert consensus 2015 made recommendations with regard to Ki67LI evaluation. Specifically, with a median Ki67LI of 20% for estrogen receptor-positive disease, values of 30% or above should be considered as high, while values of 10% or less should be regarded as low. Ki67LI standardization would improve the definition of the appropriate treatment strategy.
Using core-needle biopsy specimens (CNBSs) for determining the expression of biomarkers such as estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor type 2 (HER2), and Ki67 by immunohistochemistry may allow to define the appropriate therapeutic strategy before surgery. Moreover, in case of neoadjuvant treatment, CNBS is the only material available for molecular testing. In addition, fixation conditions should be more appropriately applied to CNBSs compared to surgically resected specimens (SRSs) (10). However, discordance in the expression of biomarkers between CNBSs and SRSs, due to tumor heterogeneity and sampling errors, has been reported (11,12). Notably, several studies have demonstrated that such discrepancies are particularly frequent in Ki67LI evaluation (11-17).
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is an imaging modality that allows to visualize the mass lesion based on increased tumor cell metabolism. Currently, PET/CT is widely used as a non-invasive examination for detection and staging of breast cancer. Several studies reported a significant correlation between Ki67LI and the maximum standardized uptake value (SUVmax) (18-22). These results suggest the possibility that, in CNBS, a combination of Ki67LI and SUVmax is a more accurate indicator of breast tumor metabolism, thus helping reduce the discordance in Ki67LI evaluation between CNBSs and SRSs.
The aim of the present study was to verify whether the discordance in Ki67LI assessment between CNBSs and SRSs can be reduced by analyzing the SUVmax obtained from pretreatment PET/CT in combination with Ki67LI. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-485).
Methods
Patients and tissues
This study was conducted according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013), and specific approval was obtained from the Ethics Committee of Shinshu University School of Medicine (Permit Number: 4258). Patients gave their written informed consent to the study, and the Ethics Committee approved this consent procedure. One hundred and eighteen invasive breast cancer specimens were obtained from patients who underwent surgery at the Shinshu University Hospital (Matsumoto, Nagano, Japan) between January 2014 and December 2015. All enrolled patients were required to have paired CNBS and SRSs. CNBSs were taken with a 16-gauge needle by an expert radiologist at the Ichinose Neurosurgical Hospital (Matsumoto, Nagano, Japan). For each lesion, at least three CNBSs were collected. The CNBSs and SRSs were formalin-fixed and paraffin-embedded. Histological tumor types of breast cancer were classified according to the WHO classification of tumors (5th edition) (23). Morphological and immunohistochemical parameters were retrieved from the pathological reports. Patients who received neoadjuvant chemotherapy or endocrine therapy, or had microinvasive carcinoma with predominant intraductal component, were excluded. For all eligible patients, clinicopathological parameters including age, tumor size, histological subtype, histological grade, and lymph node status were retrieved from clinical records. The clinicopathological features of patients are shown in Table 1.
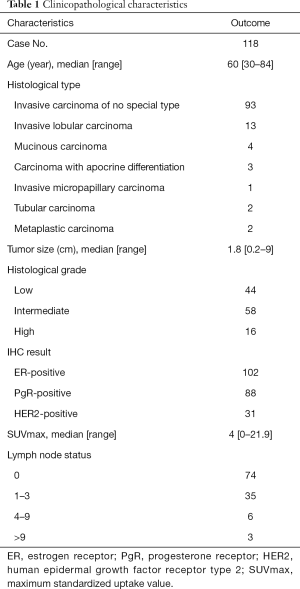
Full table
Pathological investigations
Tumors were histologically classified according to the general rules of clinical pathological recording of breast cancer (17th edition) of the Japanese Breast Cancer Society (24). Tissue samples with nuclear staining for estrogen receptor (ER) or progesterone receptor (PgR) in at least 1% of the invasive tumor cells were classified as ER or PgR-positive. HER2 was defined as positive when the tumor was scored 3+ or 2+ and and the HER2 gene was considered to be amplified if the ratio of HER2 gene to chromosome 17 signals was ≥2.0.
Immunohistochemistry and evaluation of Ki67
Immunostaining of CNBSs and SRSs for Ki67 was performed using Clone MIB-1 mouse monoclonal antibody (Dako, Glostrup, Denmark). CNB sections were stained with EnVision system (Dako), and SRS sections were stained with Ventana Bench Mark ULTRA (Roche Diagnostics, Tokyo, Japan). CNBS Ki67LI was calculated using digital image analysis (Ki67 Antigen Semi Auto Counter, SEIKOTEC CO.LTD, Fukuoka, Japan). SRS Ki67LI was calculated using visual assessment (nuclear staining was examined in approximately 500 tumor cells) by one pathologist (T.U.) blinded to the CNBS Ki67LI and clinical information. Then, we defined a concordance range by two alternative methods. In the first method, we defined ‒5%≤ ∆x ≤5% as a concordance range, where ∆x was calculated by subtracting CNBS Ki67LI from SRS Ki67LI. Here, we designated this ∆x as “(∆x) conc”. In the second method, we divided Ki67LI into three classes, i.e., Low (0≤ Ki67LI ≤10), Intermediate (10< Ki67LI <30), and High (30≤ Ki67LI). If both CNBS and SRS Ki67LI belonged to the same group, the samples were defined as concordant for that group, while if they belonged to different groups, they were defined as discordant. Thus, a concordance rate was obtained for each of the three classes by dividing the number of samples in the concordance groups by the total number of samples.
FDG-PET/CT
Patients underwent 18F-FDG PET/CT scans at the Ichinose Neurosurgical Hospital (Matsumoto, Nagano, Japan) using a standard technique. All patients were instructed to fast from 6 h before receiving an intravenous tracer injection. Ninety minutes after intravenous administration of 3.7 MBq/kg 18F-FDG, a whole-body scan from head to femoral lesion was performed in the spine position. PET/CT imaging was performed using a Discovery ST Elite Performance scanner (GE Healthcare Japan, Tokyo, Japan). Attenuation-corrected images were reconstructed in coronal planes. A region of interest (ROI) was placed in the target lesions, including the highest uptake area, and then the maximal standardized uptake value (SUVmax) in the region of interest (ROI) was calculated. Here, SUVmax = [maximal radioactivity concentration in ROI (µCi/g/injected dose (µCi)/patient’s weight (kg)]. In comparison analysis of concordance between CNBSs and SRSs, we divided the subjects into the following three groups by SUVmax: SUVmax ≤4, 4< SUVmax <8, and SUVmax ≥8.
Statistical analysis
A correlation between Ki67LI evaluated in CNB and SRS was analyzed by Spearman’s rank correlation coefficient. The correlation between CNBS and SRS Ki67LI, as well as the correlations of Ki67LI concordance and discordance groups with age, tumor size, and SUVmax were analyzed by Mann-Whitney U test. The correlation between the Ki67LI of concordance/discordance groups and histological grade, lymph node metastasis, and hormone status were analyzed by chi-square test. The correlations between the SUVmax of the concordance and discordance groups in the three Ki67LI classes, i.e., Ki67LI-Low, -Intermediate, and -High, were analyzed by Mann-Whitney U test. We examined the correlation between the SUVmax of the concordance and that of the discordance group by setting the threshold level of SUVmax to 8 for Ki67LI-High samples, and to 4 for the Ki67LI-Low class, using a chi-square test. All analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P values below 0.05 were regarded as statistically significant.
Results
Comparison of Ki67LI between CNBS and SRS
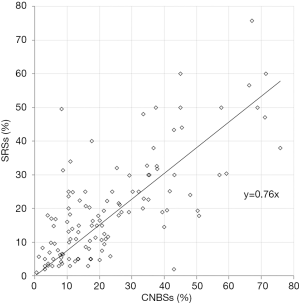
The median Ki67LI was 17.8% (0.5–75.9%) and 17.0% (1.0–75.7%) in CNBSs and SRSs, respectively. Thus, there was no difference in Ki67LI distribution between the two types of specimens (P=0.13), and a significantly positive correlation was observed between the two indexes (Figure 1, coefficient: |r|=0.76, P<0.01). When 20% was set as the threshold value for high Ki67LI level and SRS Ki67LI was set as the reference, the sensitivity, specificity, as well as positive and negative predictive Ki67LI values in CNBSs were 74.5%, 71.8%, 63.6%, and 81.0%, respectively. Consequently, the concordance rate of Ki67LI between CNBSs and SRS was 72.9% with a kappa value of 0.45.
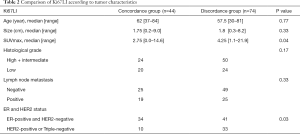
Concordance rate based on ∆x(conc) and comparison of clinicopathologic characteristics in the concordance and the discordance group
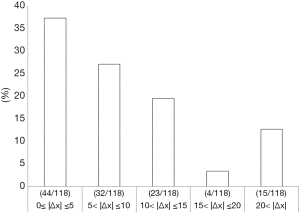
The concordance rate of Ki67LI between CNBSs and SRSs based on ∆x(conc) was 37.3% (44/118). ∆x was widely distributed, as shown in Figure 2. We compared the clinicopathological features between the Ki67LI concordance group and discordance group (Table 2). Both groups had similar clinicopathological features, including age (P=0.77), tumor size (P=0.33), histological grade (P=0.17), and lymph node metastasis (P=0.33). The Ki67LI concordance group showed a significantly lower SUVmax (median: 2.75, range 0–14.6) compared to the Ki67LI discordance group (median: 4.25, range 1.1–21.9) (P=0.04). In addition, significantly more ER-positive and HER2-negative tumors were included in the Ki67LI concordance group (P=0.03; Table 2).
Full table
Comparison of the concordance rate in the three Ki67LI groups
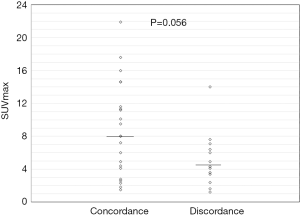
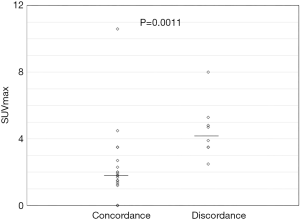
First, we compared the concordance rate between CNBSs and SRSs in the Ki67LI-Low, -Intermediate, and -High classes, and found it to be 72.4% (SRS/CNBS; 21/29), 64.8% (35/54), and 62.9% (22/35), respectively. Second, we compared the distribution of SUVmax between the concordance and the discordance groups in each of the three Ki67LI classes. Among Ki67LI-High samples, the concordance group (median: 8.0; range 1.5–21.9) showed a significantly higher SUVmax than the corresponding discordance group (median: 4.3; range 1.2–14.0; P=0.056) (Figure 3). Among Ki67LI-Intermediate samples, no difference in SUVmax distribution was observed between the concordance (median: 4.1; range 1.2–11.0) and the corresponding discordance group (median: 4.2; range 1.1–9.3) (P=0.75). Among the Ki67LI-Low samples, the concordance group (median: 1.8; range 0–10.6) showed a significantly lower SUVmax than the corresponding discordance group (median: 4.3; range 2.5–8.0; P=0.0011; Figure 4). Based on these observations, we analyzed the concordance within the Ki67LI-Low and -High classes by setting specific threshold levels for SUVmax. As a result, a significantly higher concordance rate was obtained with SUVmax ≤4 (19/23; 82.6%, P=0.033) in the concordance group within the Ki67LI-low class, and with SUVmax ≥8 (12/13; 92.3%, P=0.009) in the concordance group within the Ki67LI-high class. These concordant cases were about 30.5% (36/118) of all cases.
Discussion
Core-needle biopsy is widely used in the diagnosis of cases suspected of breast carcinoma. It is an established sampling method for histopathological diagnosis as well as immunohistochemistry examination. However, there is a concern that the diagnosis obtained from CNBSs may be less reliable than that obtained from SRSs due to smaller sample size and possible sampling errors. Several studies (11,13-17) have reported high consistency of ER, PR, and HER2 determination between preoperative CNBS and SRS. However, little evidence supports the same consistency for Ki67LI examination. Accurate determination of Ki67LI in invasive breast cancer is essential for choosing optimal neoadjuvant or adjuvant therapies. In the present study, we demonstrated that Ki67LI obtained by CNBS could be more reliable when combined with the SUVmax of tumors obtained by FDG PET/CT.
Multiple studies showed that, for ER, PgR, and HER2 statuses, the concordance between CNBSs and SRSs is about 93% to 99% (10,11,14-17), while for Ki67LI it was reported to be between 76% and 87.0% (25). However, based on ∆x(conc), we found that, although there was a significantly positive correlation in Ki67LI distribution between CNBSs and SRSs, the overall concordance rate between the two types of specimens was as low as 40%. When 20% was set as the threshold value for Ki67LI, the concordance rate increased to 74.4% with a kappa value of 0.45. Using the same threshold level, Chen et al. reported a similar result, i.e., a concordance rate of 80.4% with a kappa value of 0.60 (12). We also found that the discordance in Ki67LI determination between CNBSs and SRSs was further reduced when the SUVmax obtained from pretreatment PET/CT was analyzed in combination with Ki67LI.
Currently, FDG-PET CT imaging is widely used as a non-invasive approach for breast cancer staging (24). FDG-PET provides an index of tumor proliferation reflecting tumor glucose metabolism (26). Previous studies (18-22) showed that the Ki67LI is strongly correlated with the SUVmax. Thus, the increased energy deriving from enhanced glucose metabolism might accelerate the development of high-grade, highly proliferative tumors. Furthermore, this finding also indicated that a low SUVmax was associated with low metabolic activity in low-grade, poorly proliferative tumors. Our study further supports the association between Ki67LI and SUVmax. Notably, particularly high concordance rates were found for two specific groups of tumors: the concordance group within the Ki67LI-High class (resulting in a 92.3% rate when the SUVmax threshold was set at 8), and the concordance group within the Ki67LI-Low class, exhibiting a rate of 82.6% when the SUVmax threshold was set at 4. These findings suggested a possibility that the heterogeneity in the distribution of proliferating cells in the tumor could be low in highly proliferating tumors with high Ki67LI and high SUVmax and slowly proliferating tumors with low Ki67LI and SUVmax. Although PET/CT is not necessary in preoperative diagnosis of early breast cancer at present, PET/CT-mammography has been introduced in the diagnosis of breast cancer and concomitant evaluation of PET/CT-mammography and Ki67LI could provide more useful information. Therefore, it is clinically significant to add the information obtained by PET/CT to the preoperative evaluation.
To optimize patient care and to allow appropriate treatment de-escalation, the eighth edition of the American Joint Committee on Cancer (AJCC) staging system has recommended molecular profiling in T1/T2 tumors without lymph node metastases and ER-positive/HER2-negative status and the following four tools are recommended: Oncotype DX®, Mammaprint®, Endopredict®, and Breast Cancer Index® (27). However, none of these molecular tests is reimbursed by the national health insurance system in many European countries and Japan. Hence, the attempts to use Ki67LI as partly substitute information obtained by molecular profiling have been reported, since most of the genes evaluated by these molecular profiling assays are related to cell proliferation (28). From this point of view, we think that it is significant to utilize Ki67LI together with the information obtained from diagnostic imaging.
The main limitation of our study was the low number of samples from invasive cancers. In addition, two methods were used to calculate the Ki67LI in CNBS and SRS, i.e., image analysis software, and visual evaluation, respectively. Although recent studies reported an almost perfect correlation between the two methods (29,30), we cannot exclude that some of the observed discrepancies might be due to different measurement methods. Hence, larger numbers of patients will be needed to confirm our results.
Our results indicated that the simple comparison of Ki67LI in CNBSs and SRSs resulted in high discordance. On the other hand, this discordance could be reduced when the SUVmax obtained from pretreatment PET/CT was analyzed in combination with Ki67LI.
Acknowledgments
We thank Dr. Shinichi Tsuchiya for his informative and precise pathological diagnosis. We also thank Dr. Yutaka Imai for his radiological diagnosis. We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-485
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-485
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-485
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-485). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013), and specific approval was obtained from the Ethics Committee of Shinshu University School of Medicine (Permit Number: 4258). Patients gave their written informed consent to the study, and the Ethics Committee approved this consent procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015;24 Suppl 2:S67-72. [Crossref] [PubMed]
- de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504-13. [Crossref] [PubMed]
- Stuart-Harris R, Caldas C, Pinder SE, et al. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008;17:323-34. [Crossref] [PubMed]
- Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212-20. [Crossref] [PubMed]
- Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174-83. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Denkert C, Loibl S, Müller BM, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 2013;24:2786-93. [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Dekker TJ, Smit VT, Hooijer GK, et al. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol 2013;24:931-7. [Crossref] [PubMed]
- Chen X, Yuan Y, Gu Z, et al. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat 2012;134:957-67. [Crossref] [PubMed]
- Chen X, Zhu S, Fei X, et al. Surgery time interval and molecular subtype may influence Ki67 change after core needle biopsy in breast cancer patients. BMC Cancer 2015;15:822. [Crossref] [PubMed]
- Arnedos M, Nerurkar A, Osin P, et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol 2009;20:1948-52. [Crossref] [PubMed]
- Hodi Z, Chakrabarti J, Lee AH, et al. The reliability of assessment of oestrogen receptor expression on needle core biopsy specimens of invasive carcinomas of the breast. J Clin Pathol 2007;60:299-302. [Crossref] [PubMed]
- Lorgis V, Algros MP, Villanueva C, et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast 2011;20:284-7. [Crossref] [PubMed]
- Park SY, Kim KS, Lee TG, et al. The accuracy of preoperative core biopsy in determining histologic grade, hormone receptors, and human epidermal growth factor receptor 2 status in invasive breast cancer. Am J Surg 2009;197:266-9. [Crossref] [PubMed]
- Sutela A, Vanninen R, Sudah M, et al. Surgical specimen can be replaced by core samples in assessment of ER, PR and HER-2 for invasive breast cancer. Acta Oncol 2008;47:38-46. [Crossref] [PubMed]
- Buck A, Schirrmeister H, Kühn T, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 2002;29:1317-23. [Crossref] [PubMed]
- De Cicco C, Gilardi L, Botteri E, et al. Is [18F] fluorodeoxyglucose uptake by the primary tumor a prognostic factor in breast cancer? Breast 2013;22:39-43. [Crossref] [PubMed]
- Gil-Rendo A, Martínez-Regueira F, Zornoza G, et al. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 2009;96:166-70. [Crossref] [PubMed]
- Ito M, Shien T, Kaji M, et al. Correlation between 18F-fluorodeoxyglucose Positron Emission Tomography/computed Tomography and Clinicopathological Features in Invasive Ductal Carcinoma of the Breast. Acta Med Okayama 2015;69:333-8. [PubMed]
- Shimoda W, Hayashi M, Murakami K, et al. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 2007;14:260-8. [Crossref] [PubMed]
- Oba T, Chino T, Soma A, et al. Comparative efficacy and safety of tyrosine kinase inhibitors for thyroid cancer: a systematic review and meta-analysis. Endocr J 2020;67:1215-26. [Crossref] [PubMed]
- Oba T, Hoki T, Yamauchi T, et al. A Critical Role of CD40 and CD70 Signaling in Conventional Type 1 Dendritic Cells in Expansion and Antitumor Efficacy of Adoptively Transferred Tumor-Specific T Cells. J Immunol 2020;205:1867-77. [Crossref] [PubMed]
- You K, Park S, Ryu JM, et al. Comparison of Core Needle Biopsy and Surgical Specimens in Determining Intrinsic Biological Subtypes of Breast Cancer with Immunohistochemistry. J Breast Cancer 2017;20:297-303. [Crossref] [PubMed]
- Ueda S, Tsuda H, Saeki T, et al. Early metabolic response to neoadjuvant letrozole, measured by FDG PET/CT, is correlated with a decrease in the Ki67 labeling index in patients with hormone receptor-positive primary breast cancer: a pilot study. Breast Cancer 2011;18:299-308. [Crossref] [PubMed]
- Yamauchi T, Hoki T, Oba T, et al. CX3CR1-CD8+ T cells are critical in antitumor efficacy but functionally suppressed in the tumor microenvironment. JCI Insight 2020;5:e133920. [Crossref] [PubMed]
- Vissio E, Metovic J, Osella-Abate S, et al. Integration of Ki-67 index into AJCC 2018 staging provides additional prognostic information in breast tumours candidate for genomic profiling. Br J Cancer 2020;122:382-7. [Crossref] [PubMed]
- Maeda I, Abe K, Koizumi H, et al. Comparison between Ki67 labeling index determined using image analysis software with virtual slide system and that determined visually in breast cancer. Breast Cancer 2016;23:745-51. [Crossref] [PubMed]
- Zhong F, Bi R, Yu B, et al. A Comparison of Visual Assessment and Automated Digital Image Analysis of Ki67 Labeling Index in Breast Cancer. PLoS One 2016;11:e0150505. [Crossref] [PubMed]


