
Biparametric (bp) and multiparametric (mp) magnetic resonance imaging (MRI) approach to prostate cancer disease: a narrative review of current debate on dynamic contrast enhancement
Introduction
Prostate cancer (PCa) is the most common malignancy found in the male population and represents one of the major causes of cancer-related death (1). Since a large number of cases can be clinically silent, there is the compelling necessity of sensitive diagnostic tools for correct diagnosis and staging.
The standard clinical approach includes prostate-specific antigen (PSA) assessment, evaluation of potentially abnormal findings on digital-rectal exploration (DRE), and trans-rectal ultrasound (TRUS)-guided biopsy. Among the limitations of these methods are the increase of PSA values also under benign conditions, and the risk of not getting enough sample through TRUS-guided prostate biopsy (2,3).
In recent years, wide visibility has gained the use of multimodality imaging in cancer diseases (4-20). Magnetic resonance imaging (MRI) has shown high diagnostic accuracy, as an independent method for adequate disease rule-out, in effectively targeting the biopsy, in association with other clinical parameters such as PSA, for the evaluation of PSA density (PSAD), to increase sensitivity in identifying clinically significant (cs) lesions or cancer recurrence after specific therapy (21-32).
In the attempt to increase MRI diagnostic and prognostic validity and to limit the evaluation variability, over the years scientific and clinical communities have tried to standardize both acquisition technique and reporting.
The European Society of Urogenital Radiology (ESUR) suggests the multiparametric (mp) acquisition of the prostate using T2-weighted (T2W), diffusion weighted imaging (DWI) and dynamic contrast enhancement (DCE) sequences for all MRI examinations (33,34). The evaluation of each of these sequences translates into an objectified numerical score leading to the formulation of the Prostate Imaging Reporting and Data System (or PIRADS) (34).
The PIRADS, initially formulated in 2012, has undergone substantial changes from version 1 to version 2 in an attempt to obtain a specific algorithm to estimate the probability of malignancy of the PCa and consequently gain clinical consideration. Though its revision, also the second version of the PIRADS, however, had some limitations represented by inter- and intragroup variability (35), as described in several studies and well summarized in the systematic review issued by Stabile et al. (36).
Based on these considerations, PIRADS has lately come to the revised version 2.1, which still has some limits, represented by ambiguities and potential misclassifications of the general score, as shown by the critical analysis made by Ullrich et al. (37).
Among the most largely debated issues in the panorama of MRI of the prostate, the role of DCE remains. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/gs-20-547).
MRI of the prostate gland
MRI of the prostate is based on a zonal anatomical approach of the different glandular components (Figure 1). The prostate has four different areas divided as per McNeal’s scheme, differentiated in peripheral zone (PZ), central zone (CZ), transitional zone (TZ) and anterior fibro-muscular stroma (AFMS). These anatomical areas have different glandular structures, which result in a wide variability of the signal intensity (38).
According to these considerations, PIRADS v2.1 proposes the analysis of specific sequences considered dominant for the evaluation of these different areas (39).
T2W sequences
The high resolution T2W sequences allow excellent morphological visualization of the gland, and accurate description of its anatomy. T2W images permit also the volumetric measurement of the gland and the lesion, an accurate identification and localization of suspected PCa, and the evaluation of the extra-compartmentalization of the PCa. T2W images are also useful in guiding any targeted biopsy (40-42).
According to PIRADS recommendation, the T2W sequences are considered dominant for the detection of PCa in the TZ, which account for only 5% of glandular tissue, identifying 5 different scores based on the characteristics of signal intensity, morphology and size of the lesion (34). However, DWI sequences and the analysis of the apparent diffusion coefficient (ADC) values increase the ability to confidently evaluate the TZ, considering that homogeneous hypointense T2W signal may also be appreciated in numerous other conditions, including atrophic alterations and benign prostatic hyperplasia, outcomes of prostatitis, and post-biopsy scar areas (43).
DWI
The DWI sequences evaluate the degree of movement or diffusion of water molecules, expressing it as a parameter known as ADC. A restricted diffusion of water molecules is attributed to an increase in the cellularity of malignant lesions. Therefore, the DWI sequences provide an important quantitative biophysical parameter that directly correlates with changes in extracellular space, allowing to distinguish benign alterations from malignant lesions (44).
However, the correct selection of the b-value for the DWI is critical, depending on the latter the ability to evaluate the real restriction of water molecule diffusion. High b-values are recommended by ESUR, considering that they are better correlated with hypercellular formations, sign of the strength of the diffusion sensitizing gradient (33,34,45,46).
The ADC map provides an objective value of restriction, and has demonstrated high accuracy in identifying the degree of aggressiveness of the tumor (47-51).
The quantitative method of analysis could help less experienced readers to classify lesions and stage the degree of aggressiveness of the tumor.
DWI sequences are considered dominant in the evaluation of the PZ, which accounts for 70–75% of the glandular tissue, identifying 5 scores based on the degree of restriction and the size of the restricted area.
DCE
The term “dynamic” derives from the multiple serial images that are collected after injection of contrast media.
The primary interest linked to the use of DCE sequences is related to the significant increase in the vessels supplying the lesion which could be associated to the tumor growth.
There is a disorganized and heterogeneous formation of vessels that also present different permeability. This growth is mainly due to the release of specific molecules by the tumor cells (specific growth factors including the vascular endothelial growth factor). Numerous studies demonstrate that the higher the tumor neoangiogenesis, the worse the prognosis of the lesion.
In their study that lasted more than 20 years, Mucci et al. identified the irregularity and size of the vessels induced by the angiogenic process as a malignancy biomarker, considering that patients with small vessels had a 6-time less chance of developing lethal cancer (52).
Also, Brawer et al. identified microvascular density as an independent predictor of the tumor stage. Therefore, the quantification of tumor angiogenesis can help in stratifying the patients and planning their adequate management (53).
Among the different factors, the micro vessel density has showed excellent correlation with the DCE sequences, allowing an adequate pre-operative stratification of the lesion Gleason score with MRI, as shown by Singanamalli et al. (54).
Therefore, the clinical application of DCE-MRI for PCa is based on data demonstrating that malignant lesions show earlier and faster enhancement and earlier contrast agent washout compared with healthy prostate tissues (55). This requires fast bolus administration of contrast media combined with rapid acquisition methods.
DCE-MRI requires the use of serial 3D T1-weighted fast spoiled gradient-echo MRI sequence acquisitions before, during, and after a bolus of low-molecular-weight gadolinium contrast medium. Contrast agents in vessels and in the extracellular space shorten local relaxation times, leading to a rapid brightening of signal on the T1-weighted sequences. T1-weighted spoiled gradient-echo sequences provide high sensitivity to T1 changes, high signal-to-noise ratios, adequate anatomic coverage, and rapid data acquisition.
Ideally, the acquisitions should be obtained approximately every 5 seconds to allow the detection of early enhancement; however, many centers use acquisition times up to 15 seconds to increase the spatial resolution and identify csPCa. However, longer acquisitions (e.g., >15 seconds) are not recommended due to difficulties in identifying early enhancement, which may impair the analysis.
DCE imaging may be technically analyzed by means of qualitative or semi-quantitative method.
The qualitative analysis of DCE-MRI and its use for prostate imaging is based on the general assumption that malignant tumors show early rapid high enhancement after injection followed by a relatively rapid decline if compared to the slower and continuously increasing signal given by normal tissues during the first few minutes after contrast injection.
Unlike the visual approach, the semi-quantitative analysis calculates the kinetics of the lesion. The main method reported in literature is curve typing, which plots the kinetic of enhancement in a signal-time curve with a type 3 curve (decline after initial upslope) considered the most equivocal for PCa, especially in presence of focal asymmetric enhancing lesions.
Although the semi-quantitative approach is widely used in the assessment of DCE-MRI, limitations are reported in terms of generalization among acquisition protocols, sequences, and all the other factors contributing to the MR signal intensity, which, in turn, affects curve metrics (56).
Therefore, current recommendation of PIRADS Steering Committee does not include the routine adoption of curve analysis for prostate lesion (34).
PIRADS v2.1 proposes the presence of early enhancement among the distinctive signs of csPCa in the CZ, which accounts for 40% of the epithelial mass (other signs include glandular symmetry, the extension of the CZ beyond the verumontanum and anomalous T2 and DWI signals in comparison with the adjacent portions of AFMS).
Due to the structure of CZ, indeed, low T2 signal intensity and reduced ADC values can also be observed under normal conditions, as shown by Gupta et al., who identified CZ ADC values overlapping with those found in malignancies of other glandular portions (57). Conversely, the contrast dynamic of CZ seems to be characteristic, which can be advantageous in identifying tumors of the base (37,38,55).
DCE may be advantageous in the evaluation of AFMS also, which is the largest portion of non-glandular tissue of the prostate. AFMS usually shows low signal intensities in T2W and DWI sequences. Besides, it appears hypovascularized. However, given the close proximity of AFMS with TZ, similar considerations on signal intensity are often applied, and little value is attributed to DCE sequences.
However, the significance of angiogenesis in PCa still remains controversial (58), thus reducing the sensitivity of DCE.
Biparametric MRI (bpMRI) vs. mpMRI
Although DCE is included in the PI-RADS v2 and v2.1 guidelines, and the ESUR suggests the acquisition of all imaging sequences for prostate MR examinations, the role of DCE sequences in diagnosis and staging of PCa is rather controversial (59-61).
Recent literature indeed focuses on the overlapping diagnostic validity using bp and mp protocols in detecting csPCa (62).
The bp approach is in fact sufficient in most cases to adequately identify a negative test, without the need of studying the contrast enhancement of the prostate gland.
Similarly, T2 and DWI sequences may accurately allow to define also the degree of aggressiveness of a lesion, especially if larger or with major characteristics of malignancy (Figure 2).
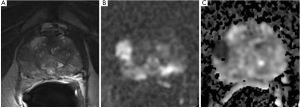
Moreover, the bp protocol offers several advantages, considering that it is time-saving and cost-effective, and does not bring the potential risks associated with contrast medium administration (63-66).
This feature would give the DCE a secondary role, probably limited to a second evaluation of the lesion location, for detecting small cancer or in case of controversy.
Small cancer detection
Recent evidence suggests that the use of DCE may increase the accuracy of prostate MRI in identifying small tumors, considering that the discriminating ability of the sequences included in the bp protocol increases with the size of the lesion, and reaches high diagnostic validity for lesions greater than 10 mm. The DCE sequences can significantly contribute to the identification of small lesions (<7 mm), whose documentation may be limited with the bp approach (67).
The capability of DCE to adequately delimitate prostate nodular lesions, even of small dimensions, could be of particular importance also for the adequate measurement of the lesion volume, which helps improve the diagnostic yield of the targeted biopsy. To our knowledge, however, there are still no studies that quantify this aspect (68).
Equivocal lesions
DCE improves the ability of stratification of patients with a PIRADS 3 or equivocal score, and helps avoid some typical pitfalls, as in the AFMS (69).
The study carried out by Greer et al. proved that DCE imaging may improve PCa detection and stratification. Notably, by analyzing the PZ, Greer et al. showed how addition of DCE to DWI determined an increase in the probability of cancer detection of 15.7%, 16.0%, and 9.2% for PIRADS category 2, 3, and 4, respectively. Lesions classified as PIRADS category 3 at DW MRI and as positive at DCE imaging in the PZ showed a higher probability of cancer detection than did DCE-negative PIRADS category 3 lesions (67.8% vs. 40.0%, P=0.02) (39).
This results in a more sensitive evaluation (70), but the clinical impact depends on how equivocal cases are managed, considering that the outcome of the examination does not change if a targeted biopsy for score 3 or more is adopted. In this perspective, as discussed also in PIRADS v2.1, the administration of contrast medium could be reserved to doubtful or suboptimal quality cases, ruling out its use in the routine scanning protocol.
This is of particular importance considering that the csPCa lesion rate in case of PIRADS 3 has been shown to be variable, ranging from 16% to 21%; these data underline the need for further stratification in these patients in whom DCE could play an important role together with other clinical/laboratory-related parameters, including the PSAD, given the risk of unnecessary biopsies (71,72).
A thorough reading of PICTURE study results reveals that functional imaging including DCE and DWI in addition to T2W images is capable to increase the number of men avoiding biopsies up to 8.9% (73).
Furthermore, as demonstrated by the PRECISION study, the PIRADS 3 rate reported in a center can be considered as a quality indicator. It should be less than 15%, considering that it tends to be proportionally higher in presence of less experienced readers (21,74).
These considerations highlight two key aspects of the biparameter approach: the experience of the reader and the image quality.
A clear example is presented by Gatti et al., who investigated the ability of six readers with different experience, divided into three groups of two readers, evaluating 1,000, 300, and 100 cases each. They interpreted 68 examinations of PCa patients, first with bpMRI including DWI and, after 1 month, with mpMRI, adding DCE. Expert readers showed excellent agreement both in bp and mp model (sensitivity =0.91–0.96, AUC =0.86–0.93; P≥0.10). DCE increased significantly the performance of both 300 and 100 case experienced radiologists, with an AUC of 0.86 and 0.77, respectively in mp model versus 0.73 and 0.68, respectively, in bp model (75). These results give additional substance to the discussion about the routine use of contrast medium as a complement to the second read of difficult cases.
On the other hand, as stated also by the recent PIRADS Committee Position paper, MRI quality is of paramount importance in the bp approach as the image quality is sufficient for detection or exclusion of csPCa (74,76).
It must be also considered, however, that lesions defined as PIRADS 4 by means of contrast enhancement in presence of equivocal cases, could be a distinct form from native PIRADS 4 lesions defined by means of bp sequences, in terms of prevalence of disease significance (Figure 3) (77).
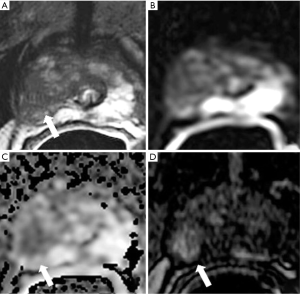
Literature on diagnostic validity
There are many studies that have investigated the diagnostic validity of the different bp and mp approaches.
In a recent meta-analysis carried out by Niu et al. from 2007 to 2017, in 33 studies on 2,383 patients, a significantly higher pooled sensitivity (0.85; 95% CI, 0.78–0.93) was reported on mpMRI compared to bpMRI (0.80; 95% CI, 0.71–0.90) (P=0.01), though evidence of a similar pooled specificity [mpMRI, 0.77 (95% CI, 0.58–0.95); bpMRI, 0.80 (95% CI, 0.64–0.96); P=0.82] (78).
Many authors, however, emphasize the overlapping diagnostic efficiency of both protocols (67,79,80) and, recently, two different meta-analyses have come to the same conclusions.
Notably, Woo et al. reported a similar pooled specificity and sensitivity between mpMRI and bpMRI in a head-to-head comparison meta-analysis including 22 studies (2,142 patients) (bpMRI: sensitivity and specificity of 0.74 and 0.90, respectively; mpMRI: sensitivity and specificity of 0.76 and 0.89, respectively) (81).
Similar accuracy was reported in the meta-analyses carried out by Alabousi et al. in 31 studies on 9,244 patients. In these studies, significant differences are reported with reference to sensitivity and specificity, considering that more robust sensitivity (around 90%) and slightly lower specificity (around 70%) were described (82).
This difference between accuracy parameters expressed in sensitivity and specificity reveals a significant variability even though the conclusions are similar (Figure 4).
High sensitivity is the true positive, because it expresses the proportion of positiveness properly identified as such, while specificity is the true negative, implying a greater likelihood of false positives with a positive test and the possible need of additional biopsies.
It is clear, therefore, that the discrepancy between the results may deeply affect the future clinical management of the patients. It is likely, however, that the described bias depends on the high heterogeneity of the studies, and recent paper of PIRADS Committee also advise caution on pooled test accuracies (77).
A different impact may have the results of the recent PROMIS study, a multicenter multi-reader trial including 497 biopsy-naïves undergoing mpMRI, which revealed no significant differences between bpMRI and mpMRI in the exclusion of csPCa, with a similar negative predictive value (90% and 91% for bpMRI and mpMRI, respectively) and sensitivity (94% and 95% for bpMRI and mpMRI, respectively), though presence of a slightly higher number of equivocal cases obtained with the bp evaluation vs. the mp one (32% vs. 28%, P=0.031). The validity of this study is mainly related to the application of the trans-perianal mapping biopsy performed independently of the MRI results, that allowed avoiding potential bias related to the use of biopsy to confirm MR findings. Moreover, even the poor comparability with other PIRADS-based studies, the use of a five-point Likert scale (as in the PROMIS study) allowed also detecting any potential advantages of DCE, unlike the PIRADS, which currently define DCE only as a dichotomous variable (68).
Recent PIRADS Committee consideration on MRI approach to naïve men with suspected PCa
Recently, the PIRADS Committee edited a narrative review including current position about MRI approach in patients with suspected PCa.
The increase in demand for prostate MRI has recently led to questioning about the preparation of the medical structures facing the increased requests (availability of scanners and experienced radiologists, able to provide accurate examinations, potentially time- and cost-sparing facilities).
Since the diagnostic performance of the bp approach was not inferior to the mp, with some exceptions, the bp model seems to be one of the possible solutions although, as overmentioned, the bp approach requires some fundamental prerequisites (i.e., high image quality and reader expertise).
As suggested by the PIRADS Committee, the current role of DCE could be limited to type 3 lesions, to determine the nature of equivocal lesions, increasing their degree and therefore the probabilities of non-benignity. Although the higher sensitivity, lower specificity may result, considering how DCE is of help in identifying a greater number of lesions, including the indolent ones.
The need therefore focuses on identifying a specific threshold of disease, which clarifies when to increase the sensitivity of the method through DCE, or when to increase the specificity, thus avoiding useless biopsies.
An adequate model to identify the clinical risk is therefore of paramount importance and include all current clinical and instrumental parameters such as PSA, anomalous findings in DRE, PSAD, or, more recently, some genomic biomarkers.
A risk model capable of determining pre-test probability of disease should allow the standardization of the MRI approach to prostate gland disease.
Low risk patients: rule-out of disease and reduced overdiagnosis of prostatic lesions are the target for low risk patients, given the low risk of PCa and therefore the high probability of negative examination. The bp approach alone may be useful once the threshold for biopsy has been established to PIRADS 4–5. In these cases, the DCE can be useful as a safety net also to evaluate low quality images.
Intermediate-to-high risk patients: including all patients with genetic predisposition or high clinical scores, patients in active surveillance for fast doubling of PSA values or with persistently elevated PSA values despite negative biopsies; for this class of patients, a mp approach should be preferred to increase the sensitivity of the method, unless in presence of lesions with typical features of malignancy. In these cases, however, the presence of the radiologist is mandatory during the acquisition.
Very high-risk patients: including patients with very high PSA values and known anomalies on DRE, suggesting a clinically significant lesion; the bp approach alone could be useful in the definitive evaluation (77).
DCE in post-treatment evaluation
DCE imaging can be used to evaluate response to therapy after radical prostatectomy (83). DCE-MRI proved to be adequate in detecting cancer recurrence when PSA begins to increase after a nadir in radical-prostatectomy patients. Detection of tumor recurrence after radical treatment can be difficult due to the lack of normal landmarks and the presence of scar tissue. In this regard, Panebianco et al. evaluated 84 patients with suspected local recurrence after prostatectomy using conventional MRI with MR spectroscopy and DCE-MRI as well as 18F-choline PET/CT and concluded that accuracy was greater for mpMRI than for PET/CT (area under the curve of MRI and PET/CT, 0.971 and 0.837, respectively) (84).
DCE-MRI is also useful in detecting recurrence after radiation therapy or ablation. Biochemical recurrence can occur in 20–40% of patients undergoing external-beam radiation therapy. Detecting recurrence after radiation therapy can be clinically challenging because the PSA level may not be a reliable marker, and the digital rectal examination can be non-specific due to fibrotic changes in the irradiated prostate gland. MpMRI with DCE sequences have shown the capacity to identify tumor recurrence with high accuracy in post-radiotherapy patients (84-86).
Conclusions
Prostate MRI is essential for detection, staging and treatment planning of csPCa. In the latest years many studies have investigated on the diagnostic accuracy of the bp approach vs. the mp one, and a debate has risen about the usefulness of DCE sequences as concrete discriminator for a definite diagnosis of csPCa.
BpMRI has proved to be non-inferior to mpMRI, although the relative superior sensitivity of mpMRI, recognizing DCE as a valuable complement in equivocal cases or smaller lesions. The bp approach needs high standard of image quality and level of expertise. Therefore, the current recommendations suggest to have both bp and mpMRI approaches available.
It remains essential to codify an appropriate decision algorithm that includes imaging and clinical-laboratory findings of the lesions, patient history and potentially promising genomic biomarkers, allowing modelling the pre-test risk of the patients and therefore standardization of the MRI approach. Further studies are necessary to investigate the DCE additional role in proper discrimination of csPCa.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Antonio Barile) for the series “Multimodality Advanced Imaging and Intervention in Gland Diseases” published in Gland Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-547
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-547
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-547). The series “Multimodality Advanced Imaging and Intervention in Gland Diseases” was commissioned by the editorial office without any funding or sponsorship. AB serves as an unpaid editorial board member of Gland Surgery from Jun 2018 to May 2022 and served as the unpaid Guest Editor of the series. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Eldred-Evans D, Neves JB, Simmons LAM, et al. Added value of diffusion-weighted images and dynamic contrast enhancement in multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer in the PICTURE trial. BJU Int 2020;125:391-8. [Crossref] [PubMed]
- Agostini A, Borgheresi A, Mari A, et al. Dual-energy CT: theoretical principles and clinical applications. Radiol Med 2019;124:1281-95. [Crossref] [PubMed]
- Borghetti P, Spiazzi L, Cozzaglio C, et al. Postoperative radiotherapy for prostate cancer: the sooner the better and potential to reduce toxicity even further. Radiol Med 2018;123:63-70. [Crossref] [PubMed]
- Loi M, Incrocci L, Desideri I, et al. Prognostic impact of nodal relapse in definitive prostate-only irradiation. Radiol Med 2018;123:631-7. [Crossref] [PubMed]
- Malaspina S, De Giorgi U, Kemppainen J, et al. 68Ga-PSMA-PET: added value and future applications in comparison to the current use of choline-PET and mpMRI in the workup of prostate cancer. Radiol Med 2018;123:952-65. [Crossref] [PubMed]
- Patel A, Jackson B. Low-dose radiation use in diagnostic imaging and cancer therapy settings. Radiol Med 2018;123:618-9. [Crossref] [PubMed]
- Petralia G, Padhani AR, Pricolo P, et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: recommendations and key uses. Radiol Med 2019;124:218-33. [Crossref] [PubMed]
- Petrillo M, Pesapane F, Fumarola EM, et al. State of the art of prostatic arterial embolization for benign prostatic hyperplasia. Gland Surg 2018;7:188-99. [Crossref] [PubMed]
- Timon G, Jereczek-Fossa BA, Fersino S, et al. Non-palliative radiotherapy in ab initio oligometastatic prostate cancer: an Italian national survey. Radiol Med 2019;124:211-7. [Crossref] [PubMed]
- Zhang H, Shen Y, Pan J, et al. MRI features after prostatic artery embolization for the treatment of medium- and large-volume benign hyperplasia. Radiol Med 2018;123:727-34. [Crossref] [PubMed]
- Compagnone G, Padovani R, D’Avanzo MA, et al. Summary of the Italian inter-society recommendations for radiation protection optimization in interventional radiology. Radiol Med 2018;123:378-84. [Crossref] [PubMed]
- De Bari B, Mazzola R, Aiello D, et al. Could 68-Ga PSMA PET/CT become a new tool in the decision-making strategy of prostate cancer patients with biochemical recurrence of PSA after radical prostatectomy? A preliminary, monocentric series. Radiol Med 2018;123:719-25. [Crossref] [PubMed]
- De Cicco L, Bracelli S. Fiducial markers implantation for prostate image-guided radiotherapy: a report on the transperineal approach. Radiol Med 2019;124:132-5. [Crossref] [PubMed]
- Fersino S, Arcangeli S, Jereczek-Fossa BA, et al. GUROPA survey: genito-urinary radiation oncology prescription attitudes. Radiol Med 2018;123:879-84. [Crossref] [PubMed]
- Filograna L, Lenkowicz J, Cellini F, et al. Identification of the most significant magnetic resonance imaging (MRI) radiomic features in oncological patients with vertebral bone marrow metastatic disease: a feasibility study. Radiol Med 2019;124:50-7. [Crossref] [PubMed]
- Barile A, Brunese L, Giovagnoni A. Gland diseases: New perspectives in diagnostic radiology. Gland Surg 2019;8:S126-9. [Crossref] [PubMed]
- Ierardi AM, Jannone ML, Brambillasca PM, et al. Bleeding after prostatectomy: Endovascular management. Gland Surg 2019;8:108-14. [Crossref] [PubMed]
- Jereczek-Fossa BA, Surgo A, Maisonneuve P, et al. Late toxicity of image-guided hypofractionated radiotherapy for prostate: non-randomized comparison with conventional fractionation. Radiol Med 2019;124:65-78. [Crossref] [PubMed]
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767-77. [Crossref] [PubMed]
- Tamada T, Sone T, Higashi H, et al. Prostate cancer detection in patients with total serum prostate-specific antigen levels of 4-10 ng/mL: Diagnostic efficacy of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging. AJR Am J Roentgenol 2011;197:664-70. [Crossref] [PubMed]
- Girometti R, Pancot M, Signor MA, et al. Multiparametric magnetic resonance imaging versus Partin tables and the Memorial Sloan-Kettering cancer center nomogram in risk stratification of patients with prostate cancer referred to external beam radiation therapy. Radiol Med 2018;123:778-87. [Crossref] [PubMed]
- Bloom JB, Gold SA, Hale GR, et al. “Super-active surveillance”: MRI ultrasound fusion biopsy and ablation for less invasive management of prostate cancer. Gland Surg 2018;7:166-87. [Crossref] [PubMed]
- Monni F, Fontanella P, Grasso A, et al. Magnetic resonance imaging in prostate cancer detection and management: a systematic review. Minerva Urol Nefrol 2017;69:567-78. [PubMed]
- Duvnjak P, Schulman AA, Holtz JN, et al. Multiparametric Prostate MR Imaging: Impact on Clinical Staging and Decision Making. Radiol Clin North Am 2018;56:239-50. [Crossref] [PubMed]
- Bhat NR, Vetter JM, Andriole GL, et al. Magnetic Resonance Imaging-Defined Prostate-Specific Antigen Density Significantly Improves the Risk Prediction for Clinically Significant Prostate Cancer on Biopsy. Urology 2019;126:152-7. [Crossref] [PubMed]
- Boesen L, Nørgaard N, Løgager V, et al. Prebiopsy Biparametric Magnetic Resonance Imaging Combined with Prostate-specific Antigen Density in Detecting and Ruling out Gleason 7-10 Prostate Cancer in Biopsy-naïve Men. Eur Urol Oncol 2019;2:311-9. [Crossref] [PubMed]
- Abdollahi H, Mofid B, Shiri I, et al. Machine learning-based radiomic models to predict intensity-modulated radiation therapy response, Gleason score and stage in prostate cancer. Radiol Med 2019;124:555-67. [Crossref] [PubMed]
- Detti B, Baki M, Becherini C, et al. High-dose intensity-modulated radiation therapy as primary treatment of prostate cancer: genitourinary/gastrointestinal toxicity and outcomes, a single-institution experience. Radiol Med 2019;124:422-31. [Crossref] [PubMed]
- Del Monte M, Leonardo C, Salvo V, et al. MRI/US fusion-guided biopsy: performing exclusively targeted biopsies for the early detection of prostate cancer. Radiol Med 2018;123:227-34. [Crossref] [PubMed]
- Faiella E, Santucci D, Greco F, et al. Analysis of histological findings obtained combining US/mp-MRI fusion-guided biopsies with systematic US biopsies: mp-MRI role in prostate cancer detection and false negative. Radiol Med 2018;123:143-52. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- PI-RADS. PI-RADS v2.1. ACR, 2019. Available online: https://www.acr.org/-/media/ACR/Files/RADS/PI-RADS/PIRADS-V2-1.pdf
- Müller S, Lilleaasen G, Sand TE, et al. Poor reproducibility of PIRADS score in two multiparametric MRIs before biopsy in men with elevated PSA. World J Urol 2018;36:687-91. [Crossref] [PubMed]
- Stabile A, Giganti F, Kasivisvanathan V, et al. Factors Influencing Variability in the Performance of Multiparametric Magnetic Resonance Imaging in Detecting Clinically Significant Prostate Cancer: A Systematic Literature Review. Eur Urol Oncol 2020;3:145-67. [Crossref] [PubMed]
- Ullrich T, Schimmöller L. Perspective: a critical assessment of PI-RADS 2.1. Abdom Radiol (NY) 2020;45:3961-8. [Crossref] [PubMed]
- Yacoub JH, Oto A. MR Imaging of Prostate Zonal Anatomy. Radiol Clin North Am 2018;56:197-209. [Crossref] [PubMed]
- Greer MD, Shih JH, Lay N, et al. Validation of the dominant sequence paradigm and role of dynamic contrast-enhanced imaging in Pi-RADS version 2. Radiology 2017;285:859-69. [Crossref] [PubMed]
- Scialpi M, D’Andrea A, Martorana E, et al. Biparametric MRI of the prostate. Turk J Urol 2017;43:401-9. [Crossref] [PubMed]
- Stanzione A, Ponsiglione A, Cuocolo R, et al. Abbreviated Protocols versus Multiparametric MRI for Assessment of Extraprostatic Extension in Prostatic Carcinoma: A multireader study. Anticancer Res 2019;39:4449-54. [Crossref] [PubMed]
- Cybulski AJ, Catania M, Brancato S, et al. Added value of MRI tractography of peri-prostatic nerve plexus to conventional T2-WI in detection of extra-capsular extension of prostatic cancer. Radiol Med 2019;124:946-54. [Crossref] [PubMed]
- Kitzing YX, Prando A, Varol C, et al. Benign conditions that mimic prostate carcinoma: MR imaging features with histopathologic correlation. Radiographics 2016;36:162-75. [Crossref] [PubMed]
- Zelhof B, Pickles M, Liney G, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int 2009;103:883-8. [Crossref] [PubMed]
- Roth Y, Tichler T, Kostenich G, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology 2004;232:685-92. [Crossref] [PubMed]
- Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: Concepts and applications. Radiographics 2009;29:1797-810. [Crossref] [PubMed]
- Manetta R, Palumbo P, Gianneramo C, et al. Correlation between ADC values and Gleason score in evaluation of prostate cancer: Multicentre experience and review of the literature. Gland Surg 2019;8:S216-22. [Crossref] [PubMed]
- Sato C, Naganawa S, Nakamura T, et al. Differentiation of noncancerous tissue and cancer lesion by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging 2005;21:258-62. [Crossref] [PubMed]
- Alessandrino F, Taghipour M, Hassanzadeh E, et al. Predictive role of PI-RADSv2 and ADC parameters in differentiating Gleason pattern 3 + 4 and 4 + 3 prostate cancer. Abdom Radiol (NY) 2019;44:279-85. [Crossref] [PubMed]
- Boesen L, Chabanova E, Løgager V, et al. Apparent diffusion coefficient ratio correlates significantly with prostate cancer gleason score at final pathology. J Magn Reson Imaging 2015;42:446-53. [Crossref] [PubMed]
- Wu X, Reinikainen P, Vanhanen A, et al. Correlation between apparent diffusion coefficient value on diffusion-weighted MR imaging and Gleason score in prostate. Diagn Interv Imaging 2017;98:63-71. [Crossref] [PubMed]
- Mucci LA, Powolny A, Giovannucci E, et al. Prospective study of prostate tumor angiogenesis and cancer-specific mortality in the health professionals follow-up study. J Clin Oncol 2009;27:5627-33. [Crossref] [PubMed]
- Brawer MK, Deering RE, Brown M, et al. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer 1994;73:678-87. [Crossref] [PubMed]
- Singanamalli A, Rusu M, Sparks RE, et al. Identifying in vivo DCE MRI markers associated with microvessel architecture and gleason grades of prostate cancer. J Magn Reson Imaging 2016;43:149-58. [Crossref] [PubMed]
- Engelbrecht MR, Huisman HJ, Laheij RJF, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology 2003;229:248-54. [Crossref] [PubMed]
- Carlani M, Mancino S, Bonanno E, et al. Combined morphological, [1H]-MR spectroscopic and contrast-enhanced imaging of human prostate cancer with a 3-Tesla scanner: Preliminary experience. Radiol Med 2008;113:670-88. [Crossref] [PubMed]
- Gupta RT, Kauffman CR, Garcia-Reyes K, et al. Apparent diffusion coefficient values of the benign central zone of the prostate: Comparison with low- and high-grade prostate cancer. AJR Am J Roentgenol 2015;205:331-6. [Crossref] [PubMed]
- Russo G, Mischi M, Scheepens W, et al. Angiogenesis in prostate cancer: onset, progression and imaging. BJU Int 2012;110:E794-E808. [Crossref] [PubMed]
- Girometti R, Cereser L, Bonato F, et al. Evolution of prostate MRI: from multiparametric standard to less-is-better and different-is better strategies. Eur Radiol Exp 2019;3:5. [Crossref] [PubMed]
- Becker AS, Kirchner J, Sartoretti T, et al. Interactive, Up-to-date Meta-Analysis of MRI in the Management of Men with Suspected Prostate Cancer. J Digit Imaging 2020;33:586-94. [Crossref] [PubMed]
- Beyhan M, Sade R, Koc E, et al. The evaluation of prostate lesions with IVIM DWI and MR perfusion parameters at 3T MRI. Radiol Med 2019;124:87-93. [Crossref] [PubMed]
- Cosma I, Tennstedt-Schenk C, Winzler S, et al. The role of gadolinium in magnetic resonance imaging for early prostate cancer diagnosis: A diagnostic accuracy study. PLoS One 2019;14:e0227031. [Crossref] [PubMed]
- Porter KK, King A, Galgano SJ, et al. Financial implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis 2020;23:88-93. [Crossref] [PubMed]
- Ward R, Purysko AS. Round table: arguments against using multiparametric prostate MRI protocols. Abdom Radiol (NY) 2020;45:3997-4002. [Crossref] [PubMed]
- van der Leest M, Israël B, Cornel EB, et al. High Diagnostic Performance of Short Magnetic Resonance Imaging Protocols for Prostate Cancer Detection in Biopsy-naïve Men: The Next Step in Magnetic Resonance Imaging Accessibility. Eur Urol 2019;76:574-81. [Crossref] [PubMed]
- Junker D, Steinkohl F, Fritz V, et al. Comparison of multiparametric and biparametric MRI of the prostate: are gadolinium-based contrast agents needed for routine examinations? World J Urol 2019;37:691-9. [Crossref] [PubMed]
- Scialpi M, Prosperi E, D’andrea A, et al. Biparametric versus multiparametric mri with non-endorectal coil at 3t in the detection and localization of prostate cancer. Anticancer Res 2017;37:1263-71. [Crossref] [PubMed]
- Bosaily AE, Frangou E, Ahmed HU, et al. Additional Value of Dynamic Contrast-enhanced Sequences in Multiparametric Prostate Magnetic Resonance Imaging: Data from the PROMIS Study. Eur Urol 2020;78:503-11. [Crossref] [PubMed]
- Rosenkrantz AB, Verma S, Turkbey B. Prostate cancer: top places where tumors hide on multiparametric MRI. AJR Am J Roentgenol 2015;204:W449-W456. [Crossref] [PubMed]
- Zawaideh JP, Sala E, Shaida N, et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: assessment of contrast benefit in clinical practice. Eur Radiol 2020;30:4039-49. [Crossref] [PubMed]
- Xu L, Zhang G, Shi B, et al. Comparison of biparametric and multiparametric MRI in the diagnosis of prostate cancer. Cancer Imaging 2019;19:90. [Crossref] [PubMed]
- Boesen L, Thomsen FB, Nørgaard N, et al. A predictive model based on biparametric magnetic resonance imaging and clinical parameters for improved risk assessment and selection of biopsy-naïve men for prostate biopsies. Prostate Cancer Prostatic Dis 2019;22:609-16. [Crossref] [PubMed]
- Simmons LAM, Kanthabalan A, Arya M, et al. The PICTURE study: Diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br J Cancer 2017;116:1159-65. [Crossref] [PubMed]
- Giganti F, Allen C, Emberton M, et al. Prostate Imaging Quality (PI-QUAL): A New Quality Control Scoring System for Multiparametric Magnetic Resonance Imaging of the Prostate from the PRECISION trial. Eur Urol Oncol 2020;3:615-9. [Crossref] [PubMed]
- Gatti M, Faletti R, Calleris G, et al. Prostate cancer detection with biparametric magnetic resonance imaging (bpMRI) by readers with different experience: performance and comparison with multiparametric (mpMRI). Abdom Radiol (NY) 2019;44:1883-93. [Crossref] [PubMed]
- de Rooij M, Israël B, Tummers M, et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists' training. Eur Radiol 2020;30:5404-16. [Crossref] [PubMed]
- Schoots IG, Barentsz JO, Bittencourt LK, et al. PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy Naive Men with Suspected Prostate Cancer: A Narrative Review. AJR Am J Roentgenol 2021;216:3-19. [Crossref] [PubMed]
- Niu XK, Chen XH, Chen ZF, et al. Diagnostic Performance of Biparametric MRI for Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2018;211:369-78. [Crossref] [PubMed]
- Choi MH, Kim CK, Lee YJ, et al. Prebiopsy biparametric MRI for clinically significant prostate cancer detection with PI-RADS version 2: A multicenter study. AJR Am J Roentgenol 2019;212:839-46. [Crossref] [PubMed]
- Brancato V, Di Costanzo G, Basso L, et al. Assessment of DCE Utility for PCa Diagnosis Using PI-RADS v2.1: Effects on Diagnostic Accuracy and Reproducibility. Diagnostics (Basel) 2020;10:164. [Crossref] [PubMed]
- Woo S, Suh CH, Kim SY, et al. Head-to-Head Comparison Between Biparametric and Multiparametric MRI for the Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2018;211:W226-W241. [Crossref] [PubMed]
- Alabousi M, Salameh JP, Gusenbauer K, et al. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis. BJU Int 2019;124:209-20. [Crossref] [PubMed]
- Verma S, Turkbey B, Muradyan N, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol 2012;198:1277-88. [Crossref] [PubMed]
- Panebianco V, Sciarra A, Lisi D, et al. Prostate cancer: 1HMRS-DCEMR at 3 T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur J Radiol 2012;81:700-8. [Crossref] [PubMed]
- Ménard C, Iupati D, Publicover J, et al. MR-guided prostate biopsy for planning of focal salvage after radiation therapy. Radiology 2015;274:181-91. [Crossref] [PubMed]
- Rouvière O, Valette O, Grivolat S, et al. Recurrent prostate cancer after external beam radiotherapy: Value of contrast-enhanced dynamic MRI in localizing intraprostatic tumor - Correlation with biopsy findings. Urology 2004;63:922-7. [Crossref] [PubMed]




