
A single-center retrospective analysis of childhood hepatoblastoma in China
Introduction
Primary liver cancer is a rare disease in children globally, accounting for 1–2% of all pediatric cancers (1-3). The most common primary liver cancer is hepatoblastoma (HB), which makes up approximately 50–80% (3-10), and with a much higher proportion (91%) among children under 5 years old (8,11,12).
The incidence rate of pediatric HB varies in different countries and regions and is more common in infants (4). The incidence rate of HB in the United States (4), in Europe (13), and South Africa (14) was 0.8–1.5, 1.2, and 0.61 per million children, respectively. For survival, the survival rate of HB has passed 70% (15-17). A study in the US showed the 1-, 5-, and 10-year survival for HB was 81.5%, 71.6%, and 69%, respectively (18). Another study in Europe found that the 5-year survival rate for HB was 66% (13). It was higher for patients undergoing liver transplant or resection. The 1-, 5-, and 10-year survival rate for HB was 93%, 82%, and 82%, respectively (19).
However, compared with studies in epidemiology, primary, and clinical medicine globally, there are only a few studies in China, especially in mainland China. A multi-center study on 153 HB children reported that the 6-year survival rate was 83.3%±3.1% (15). However, results of single-center studies varied. A study in Shanghai on 104 children with HB reported the 5-year survival rate of 86.3±5.0% (20). A study in Beijing on 176 children with HB reported that the 5-year survival rate was 54.6% (21). Another study in Beijing on 102 children with HB found that advanced HB was associated with a low survival rate, from 75.0% in stage II to 20.2% in stage IV (22). In addition, studies in Hong Kong and Taiwan always concentrated on the comparisons between HB and hepatocellular carcinoma (23,24).
Therefore, considering the scarcity of studies in China, this study aimed to describe the epidemiological characteristics and analyze the influencing factors of survival among children with HB in mainland China, which would provide some evidence for the improvement of prognosis for children with HB. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-710).
Methods
Inclusion and exclusion criteria
This study retrospectively reviewed 115 children with HB treated in Renji Hospital Affiliated to the Shanghai Jiao Tong University School of Medicine, Shanghai, China, from January 2013 to December 2019. However, 87 patients were enrolled in this study. The enrollment criteria in this study were: (I) patients should be younger than 18 years old; (II) patients should be dragonized as HB; (III) patients or their parents should participate in this study. The exclusion criteria were: (I) patients or their parents refused to be followed up; (II) patients did not accept any treatment.
The Ethics Committee approved the study protocol of the Shanghai Jiao Tong University School of Medicine. The written informed consent of all enrolled patients or their parents was obtained. Patient anonymity was guaranteed to be preserved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Diagnosis and treatment
The diagnosis was on clinical manifestations, laboratory examinations, and pathological examinations. Patients were treated with hepatectomy, liver transplantation, or without surgery on their conditions. Most of the patients not undergoing surgery were hospitalized for biopsy and clinical evaluations and did not experience further surgical treatment.
Follow-up
The last follow-up date was December 31, 2019. Follow-ups were conducted after patients discharged from the hospital. Overall survival (OS) was estimated as time (in days) from diagnosis to death of any cause. Patients not experiencing an event were censored from the last contact.
Statistical analysis
Two researchers entered all the data simultaneously. Data collection and analysis were performed using SPSS 18.0 (Statistical Package for the Social Science, SPSS Inc., Chicago, IL, USA). Descriptive analysis was used to describe the demographic and disease characteristics. Then, the continuous variables were described as mean ± SEM. Categorical variables are described by number and percentage. Survival time, survival rates, and survival curves were estimated by the Kaplan-Meier method. A log-rank test was used in the univariate analysis. Variables statistically significant in the univariate analysis, and variables with professional meanings on previous publications, were included in the Cox proportional hazards model. All tests were two-way, and P<0.05 was considered statistically significant.
Results
Patients’ characteristics
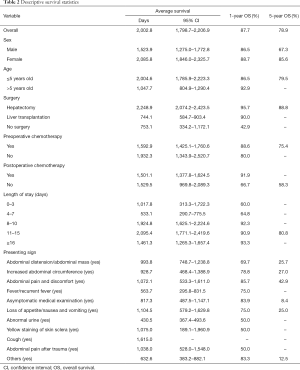
Eighty-seven children were recruited into this study from Shanghai Renji Hospital. The primary characteristics of the patients were summarized in Table 1. The sex distribution was 1:1.23 (M:F). The average age at diagnosis was 3.0±0.3 years old (range, 0.0 to 12.0 years old), of which the majority (81.6%) were under 5 years old. More than half (64.4%) of patients underwent hepatectomy, 19.5% had liver transplantation, and 16.1% did not experience any surgery. Most patients (88.5%) underwent chemotherapy before surgery, including those without surgery, while fewer patients (82.8%) underwent chemotherapy after surgery. The average lengths of stay were 16.3±1.1 days (range, 1 to 55 days), and a half (42.5%) stayed in the hospital at least 16 days. The most frequent presenting sign was abdominal distension or abdominal mass (36.8%). The average medical expenses were 40,217.5±3,862.0 CNY (Chinese Yuan; ~US$5,745.4±US$551.7).

Full table
Survival descriptions of different subgroups
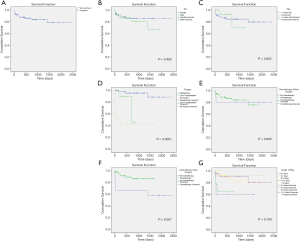
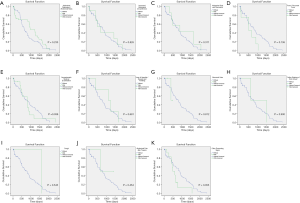
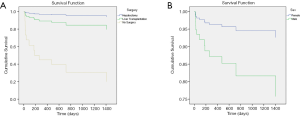
For all 87 patients, the overall average survival was 2,002.8 days (95% CI: 1,798.7–2,206.9 days), the 1-year OS and the 5-year OS were 87.7% and 78.9%, respectively (Table 2 and Figure 1A). Descriptive survival statistics and Kaplan-Meier curves showed that surgery and chemotherapy after surgery had prognostic significance. The 1-year OS rates of boys and girls were 86.5% and 88.7% (P=0.485), respectively; the 5-year OS rates were 67.3% and 85.6%, respectively (Table 2 and Figure 1B). The 1-year OS for patients under 5 years old was 86.5% and 92.9% in patients over 5 years old (Table 2 and Figure 1C, P=0.852). Undergoing hepatectomy was associated with an increasing average survival from 753.1 to 2,248.9 days and a 1-year OS rate from 42.9% to 95.7%; liver transplantation was associated with an increasing 1-year OS rate from 42.9% to 90.0% (Table 2 and Figure 1D, P<0.0001). The 1-year OS rate for patients with preoperative chemotherapy was 88.6%, and 80.0% for those without (Table 2 and Figure 1E, P=0.965). Patients having postoperative chemotherapy are associated with an increasing 1-year OS rate from 66.7% to 91.9% (Table 2 and Figure 1F, P=0.007). The 1-year OS rates for patients staying in hospital for 0–3, 4–7, 8–10, 11–15, and ≥16 days were 60.0%, 64.8%, 92.3%, 90.9%, and 93.3%, respectively (Table 2 and Figure 1G, P=0.100). The average survival rates, the 1- and 5-year OS, for patients with various presenting signs were shown in Table 2 and Figure 2 (all P values >0.05).
Full table
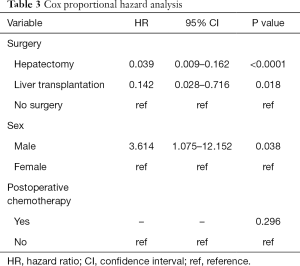
Cox proportional hazard analysis
Factors with statistical significance in the univariate analysis were included in the Cox proportional hazard model. Sex was chosen on existing studies (15). The multivariate analysis suggested that undergoing surgery and sex were independent prognostic factors of childhood HB (Table 3, P<0.05). Undergoing hepatectomy (HR: 0.039, Figure 3A) or liver transplantation (HR: 0.142, Figure 3A) was associated with a better prognosis, while boys were associated with a poorer prognosis (HR: 3.614, Figure 3B).
Full table
Comparison of the medical expenses of different surgical approaches
Surgery could lead to a better prognosis, and patients with hepatectomy had better outcomes than those with liver transplantation. Therefore, it would be meaningful for parents and doctors to consider more about medical expenses when determining the treatment for children with HB because the economic burden was always an essential factor impeding the treatment effect. For patients undergoing hepatectomy, their average medical expenses were 30,167.1±1,575.0 CNY (~US$4,309.6±US$225.0); for those undergoing liver transplantation, their average medical expenses were 99,633.9±9,154.3 CNY (~US$14,233.4±US$1,307.8); for those not having surgery, their average medical expenses were 8,270.7±2,566.2 CNY (~US$1,181.5±US$366.6). Also, because the Levene test showed the uneven variance (P<0.0001), the Kruskal-Wallis H test was used to explore the difference between the medical expenses of the three groups. Statistics suggested that the medical expenses of the three groups were statistically different (P<0.0001). The Mann-Whitney U test showed that the medical expenses between hepatectomy and liver transplantation (P<0.0001), hepatectomy, and no surgery (P<0.0001), liver transplantation, and no surgery (P<0.0001) were all statistically significant (Table 4).

Full table
Discussion
In the past four decades, the diagnosis and treatment of pediatric HB have developed a lot globally (25,26), which led to a clear increase in the survival rate of children with HB. Statistics in the 1980s showed that the 5-year survival rate for children with HB was 36% (27). However, combined with modern chemotherapy and surgical technologies (16,28,29), the 5-year survival rate in the United States from 1983 to 2005 was 76% (27), and in Spain, the 5-year survival rate from 1991 to 2009 was 73.2% (30). Consistent with global trends, this study in China from 2013 to 2019 showed that the 5-year OS was 78.9%, which was comparable with other single-center studies nationwide. A single-center study in 2014 in Beijing suggested that the 5-year OS was 66.7% (21). However, the 5-year OS in another single-center study in Shanghai from 2011 to 2017 was 86.3% (20). It was like the 5-year OS of 77.7% from a Hong Kong study from 1996 to 2014 (31).
To explore the factors affecting the prognosis of childhood HB, the results suggested that undergoing surgery, including hepatectomy and liver transplantation, was associated with a better prognosis. Earlier studies have dug a lot in hepatectomy and liver transplantation. Surgical therapy plays a significant role in improving prognosis, especially hepatectomy, combined with chemotherapy. Although over 60% of tumors appear unresectable at the beginning (16), approximately 40% can be resected after chemotherapy (32-34). For the left 20% of tumors, liver transplantation is the best solution (35), which provides a potential cure opportunity for children with advanced HB (36,37). Both surgery therapies improved the survival rate for children with HB in various stages. The results showed that the 1- and 5-year OS rates for children with hepatectomy were 95.7% and 88.8%, respectively, and the 1-year OS for children with liver transplantation was 90.0%, which was following previous research. Several studies in the 1990s showed the long-term survival rates for children with hepatectomy varied from 63% to 94% (38-41); another study from 1990 to 2013 in the Netherlands suggested children undergoing hepatectomy had the 5-year survival of 92% (42). For children undergoing liver transplantation, the 1-year survival rates in the United States varied from 79% to 92% (36,37,43); the survival rates in different countries ranged from 62.5% to 90% (37). Therefore, it was highly recommended that children with HB be treated by hepatectomy or liver transplantation. To further explore the two surgical therapies, their combination with chemotherapy contributed significantly to the improvement of survival (44). Preoperative chemotherapy can help more children be suitable for hepatectomy (4,37,45), and postoperative chemotherapy can dramatically improve the survival rate, whether for hepatectomy or liver transplantation (32,33). Although the results and some other studies suggested whether receiving chemotherapy before or after surgery did not show significant influence on prognosis (46,47), the reason could not be attributed to chemotherapy hastily, and routine chemotherapy should be conducted according to treatment guidelines (48,49). The guidelines also suggest that postoperative chemotherapy can completely or partially relieve intrahepatic metastasis and recurrence of hepatoblastoma caused by postoperative residual. The sample size should be expanded further to testify these effects.
Also, although international studies showed that male children were more sensitive to HB, there was no evidence verifying the influence of sex on survival (17,50). However, considering a Chinese study suggested that a male was an individual factor contributing to poorer prognosis (15), sex was included in the multivariate analysis even though it was not statistically significant in the univariate analysis. Like this previous publication in China, our study showed that sex was an influencing factor of prognosis, and boys had a poorer prognosis than girls. On this finding, it is necessary to explore further the relationship between sex and prognosis of childhood HB. The differences among various countries and regions should be further considered.
Almost no studies ever reported medical expenses of childhood HB. However, it should be noted that the economic burden is always a significant obstacle for patients to receive effective therapy (51), and there is no exception for children with HB (15). This study showed that the most expensive therapy was liver transplantation (99,633.9 CNY), followed by hepatectomy (40,217.5 CNY) and no surgery (8,270.7 CNY). Compared with the real GDP per capita of 70,892 CNY, the per capita disposable income for urban residents of 42,359 CNY, and the per capita disposable income for rural residents of 16,021 CNY in China in 2019, it posed an economic burden for Chinese families with children suffering from HB. Therefore, it should be given severe considerations when accelerating the survival of children with HB. Further cost-effectiveness analysis on diagnosis and treatment is worth being done (52), which will provide more evidence for the government to invest more on child health and encourage children with HB to receive more effective therapies.
Although a retrospective analysis is suitable for rare diseases with advantages of time- and cost-saving, there are some biases affecting the results, especially selection bias. To avoid Berkson bias as much as possible, this study includes all children with HB meeting the inclusion and exclusion criteria treated in Renji Hospital from 2013 to 2019. Their survival and death information are tried to be extracted from medical records to avoid McNeyman bias and non-response bias. However, this retrospective study has several limitations. First, because of the accessibility of data, some traditional key clinical characteristics, histology, pathology, and staging were not included in this study, including AFP level at diagnosis, histopathology, PRETEXT stage, and metastasis. Second, this is a single-center study with small samples, and the representativeness is limited. Third, it is a short-term follow-up study, and many participants were censored because they are still alive at the last follow-up. Fourth, considering the economic burden of different surgical therapies, only the average medical expenses were presented and compared rather than a more comprehensive analysis combined with health utilities. Fifth, considering the characteristics of a retrospective study that it is impossible to analyze the causal relationship, the imaging features and pathogenesis of HB was not explored in this study. Therefore, further study, including more factors and participants from various institutions, needs to be designed into a prospective research to verify the results. And a cost-effectiveness analysis should be considered in the next step.
Conclusions
In conclusion, this study showed that surgical therapy could bring a better prognosis, and girls had excellent opportunities for survival. Although some of these study findings have been reported previously, this study could provide some significant evidence nationwide, especially some conflicting factors and from the aspect of economic burden.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China [2017YFC0908100]; and the National Natural Science Foundation of China [81902939].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-710
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-710
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-710). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee approved the study protocol of the Shanghai Jiao Tong University School of Medicine. The written informed consent of all enrolled patients or their parents was obtained. Patient anonymity was guaranteed to be preserved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly D, Sharif K, Brown RM, et al. Hepatocellular carcinoma in children. Clin Liver Dis 2015;19:433-47. [Crossref] [PubMed]
- Feng TC, Zai HY, Jiang W, et al. Survival and analysis of prognostic factors for hepatoblastoma: based on SEER database. Ann Transl Med 2019;7:555. [Crossref] [PubMed]
- Raney B. Hepatoblastoma in children: a review. J Pediatr Hematol Oncol 1997;19:418-22. [Crossref] [PubMed]
- Herzog CE, Andrassy RJ, Eftekhari F. Childhood cancers: hepatoblastoma. Oncologist 2000;5:445-53. [Crossref] [PubMed]
- Khanna R, Verma SK. Pediatric hepatocellular carcinoma. World J Gastroenterol 2018;24:3980-99. [Crossref] [PubMed]
- Darbari A, Sabin KM, Shapiro CN, et al. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003;38:560-6. [Crossref] [PubMed]
- Mann JR, Kasthuri N, Raafat F, et al. Malignant hepatic tumours in children: incidence, clinical features and aetiology. Paediatr Perinat Epidemiol 1990;4:276-89. [Crossref] [PubMed]
- Moore SW, Davidson A, Hadley GP, et al. Malignant liver tumors in South African children: a national audit. World J Surg 2008;32:1389-95. [Crossref] [PubMed]
- Gururangan S, O'Meara A, MacMahon C, et al. Primary hepatic tumours in children: a 26-year review. J Surg Oncol 1992;50:30-6. [Crossref] [PubMed]
- Pham TH, Iqbal CW, Grams JM, et al. Outcomes of primary liver cancer in children: an appraisal of experience. J Pediatr Surg 2007;42:834-9. [Crossref] [PubMed]
- Fernandez-Pineda I, Cabello-Laureano R. Differential diagnosis and management of liver tumors in infants. World J Hepatol 2014;6:486-95. [Crossref] [PubMed]
- Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 2014;26:19-28. [Crossref] [PubMed]
- Stiller CA, Pritchard J, Steliarova-Foucher E. Liver cancer in European children: incidence and survival, 1978-1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2115-23. [Crossref] [PubMed]
- Moore SW, Millar AJ, Hadley GP, et al. Hepatocellular carcinoma and liver tumors in South African children: a case for increased prevalence. Cancer 2004;101:642-9. [Crossref] [PubMed]
- Yuan XJ, Wang HM, Jiang H, et al. Multidisciplinary effort in treating children with hepatoblastoma in China. Cancer Lett 2016;375:39-46. [Crossref] [PubMed]
- Perilongo G, Shafford E, Plaschkes J, et al. SIOPEL trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol 2000;1:94-100. [Crossref] [PubMed]
- Schnater JM, Aronson DC, Plaschkes J, et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 2002;94:1111-20. [Crossref] [PubMed]
- Vinayak R, Cruz RJ Jr, Ranganathan S, et al. Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981-2015). Liver Transpl 2017;23:1577-88. [Crossref] [PubMed]
- Pham TA, Gallo AM, Concepcion W, et al. Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surg 2015;150:1150-8. [Crossref] [PubMed]
- Wang TY, Han YL, Gao YJ, et al. Retrospective Analysis of Childhood Hepatoblastoma in a Single Centre in China. Clin Oncol (R Coll Radiol) 2019;31:471-8. [Crossref] [PubMed]
- Qiao GL, Chen Z, Wang C, et al. Pure fetal histology subtype was associated with better prognosis of children with hepatoblastoma: A Chinese population-based study. J Gastroenterol Hepatol 2016;31:621-7. [Crossref] [PubMed]
- Zhang Y, Zhang W, Tang S, et al. A single-center retrospective study of pediatric hepatoblastoma. Oncol Lett 2016;12:3919-25. [Crossref] [PubMed]
- Chan KL, Fan ST, Tam PK, et al. Paediatric hepatoblastoma and hepatocellular carcinoma: retrospective study. Hong Kong Med J 2002;8:13-7. [PubMed]
- Lee CL, Ko YC. Survival and distribution pattern of childhood liver cancer in Taiwan. Eur J Cancer 1998;34:2064-7. [Crossref] [PubMed]
- Hiyama E. Current therapeutic strategies for childhood hepatic malignant tumors. Int J Clin Oncol 2013;18:943-5. [Crossref] [PubMed]
- Kremer N, Walther AE, Tiao GM. Management of hepatoblastoma: an update. Curr Opin Pediatr 2014;26:362-9. [Crossref] [PubMed]
- Horton JD, Lee S, Brown SR, et al. Survival trends in children with hepatoblastoma. Pediatr Surg Int 2009;25:407-12. [Crossref] [PubMed]
- Meyers RL, Tiao GM, Dunn SP, et al. Liver transplantation in the management of unresectable hepatoblastoma in children. Front Biosci (Elite Ed) 2012;4:1293-302. [Crossref] [PubMed]
- Evans AE, Land VJ, Newton WA, et al. Combination chemotherapy (vincristine, adriamycin, cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer 1982;50:821-6. [Crossref] [PubMed]
- Barrena S, Hernandez F, Miguel M, et al. High-risk hepatoblastoma: results in a pediatric liver transplantation center. Eur J Pediatr Surg 2011;21:18-20. [Crossref] [PubMed]
- Liu APY, Ip JJK, Leung AWK, et al. Treatment outcome and pattern of failure in hepatoblastoma treated with a consensus protocol in Hong Kong. Pediatr Blood Cancer 2019;66:e27482. [Crossref] [PubMed]
- Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 2000;36:1418-25. [Crossref] [PubMed]
- Hishiki T. Current therapeutic strategies for childhood hepatic tumors: surgical and interventional treatments for hepatoblastoma. Int J Clin Oncol 2013;18:962-8. [Crossref] [PubMed]
- Watanabe K. Current chemotherapeutic approaches for hepatoblastoma. Int J Clin Oncol 2013;18:955-61. [Crossref] [PubMed]
- Molmenti EP, Wilkinson K, Molmenti H, et al. Treatment of unresectable hepatoblastoma with liver transplantation in the pediatric population. Am J Transplant 2002;2:535-8. [Crossref] [PubMed]
- Reyes JD, Carr B, Dvorchik I, et al. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 2000;136:795-804. [Crossref] [PubMed]
- Austin MT, Leys CM, Feurer ID, et al. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 2006;41:182-6. [Crossref] [PubMed]
- Carceller A, Blanchard H, Champagne J, et al. Surgical resection and chemotherapy improve survival rate for patients with hepatoblastoma. J Pediatr Surg 2001;36:755-9. [Crossref] [PubMed]
- Ehrlich PF, Greenberg ML, Filler RM. Improved long-term survival with preoperative chemotherapy for hepatoblastoma. J Pediatr Surg 1997;32:999-1002; discussion 1003. [Crossref] [PubMed]
- King DR, Ortega J, Campbell J, et al. The surgical management of children with incompletely resected hepatic cancer is facilitated by intensive chemotherapy. J Pediatr Surg 1991;26:1074-80; discussion 1080-1. [Crossref] [PubMed]
- Reynolds M, Douglass EC, Finegold M, et al. Chemotherapy can convert unresectable hepatoblastoma. J Pediatr Surg 1992;27:1080-3; discussion 1083-4. [Crossref] [PubMed]
- Busweiler LA, Wijnen MH, Wilde JC, et al. Surgical treatment of childhood hepatoblastoma in the Netherlands (1990-2013). Pediatr Surg Int 2017;33:23-31. [Crossref] [PubMed]
- Ezekian B, Mulvihill MS, Schroder PM, et al. Improved contemporary outcomes of liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma. Pediatr Transplant 2018;22:e13305. [Crossref] [PubMed]
- Hafberg E, Borinstein SC, Alexopoulos SP. Contemporary management of hepatoblastoma. Curr Opin Organ Transplant 2019;24:113-7. [Crossref] [PubMed]
- Krstovski N, Janic D, Dokmanovic L, et al. Clinical characteristics and survival of children with hepatoblastoma--single centre experience. Srp Arh Celok Lek 2008;136:603-8. [Crossref] [PubMed]
- Kaliciński P, Otte JB. Liver transplantation for hepatic malignancies in children--we all need more information. Pediatr Transplant 2009;13:657-8. [Crossref] [PubMed]
- Buckel E, Silva G, Brahm J, et al. Experience of a single center in liver transplantation in adults and children. Revista Medica De Chile 1996;124:27-36. [PubMed]
- Kirnap M, Ayvazoglu Soy E, Ozcay F, et al. Pediatric liver transplant for hepatoblastoma: a single-center experience. Exp Clin Transplant 2017;15:50-2. [PubMed]
- Compilation and Examination Expert Group for Guidelines for the diagnosis and treatment of hepatoblastoma (2019). Guidelines for the diagnosis and treatment of hepatoblastoma (2019). J Clin Hepatol 2019;35:2431-4.
- Perilongo G, Shafford E, Maibach R, et al. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 2004;40:411-21. [Crossref] [PubMed]
- Warner EL, Kirchhoff AC, Nam GE, et al. Financial burden of pediatric cancer for patients and their families. J Oncol Pract 2015;11:12-8. [Crossref] [PubMed]
- Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer 2012;59:818-21. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


