
Breast implant illness: a topic in review
Breast implant history
Since their introduction in 1962 by Cronin and Gerow, the safety of silicone breast implants has been studied more closely than many available medical devices. Today, nearly 300,000 breast augmentations and 100,000 breast reconstructions utilize breast implants annually in the United States (US). Despite years of technological advancement in the design and manufacturing, the basic design largely remains the same (1-3).
In the early 1980’s, just as third-generation silicone implants were being introduced, rising levels of consumer concern became evident in regard to the safety of silicone breast implants. During this time, the FDA’s new surveillance system began to identify frequent local complications associated with silicone implants and these devices became classified as higher-risk devices. This new label required manufactures to become responsible for providing data that supported the safety of these devices for patient use (4). The safety of silicone breast implants has been controversial, with >400 reports on various health conditions believe to be in association with breast implants (5).
Silicone breast implants were removed from market in 1992 after the FDA concluded that breast implant manufacturers had failed to appropriately address safety concerns. At that time manufacturers were instructed to perform core studies to better assess the safety profiles of silicone breast implants (6-10). Seven years later, the Institute of Medicine (IOM) released a report titled, “Safety of Silicone Breast Implants”, which highlighted that most concerning are local complications and that in order to conclude implant safety in regard to systemic disease, further well controlled large sample size studies were indicated (4).
The IOM brought clarity to what scientific evidence existed at that time, and it identified safety information gaps. This report was instrumental in bringing silicone breast implants back to market in 2006. Since their return, the scientific community continued to conduct extensive research in order to further address breast implant safety. This is largely in response to an FDA mandate that that the two manufacturers of silicone breast implants at that time, Allergan Inc. (Dublin, IE, Ireland) and Mentor Corp (Minneapolis, MN, USA) conduct large post-approval studies to guarantee that these potential long-term risks did not go unmonitored (11,12).
Current state of affairs
Continued research has greatly expanded our current knowledge on silicone breast implant safety concerns since the FDA-mandated moratorium in the 1990’s (4,13). Plastic surgeons must hold industry and each other accountable for the care of their patients by increasing awareness of evidence-based practices so that they can best inform their patients and the medical community on the safety of these devices (12,14,15). Plastic surgeons, like all physicians, took a Hippocratic oath to “do no harm” and have a responsibility to best inform their patients on the safety of these devices and to listen with a kind ear when patients present with symptoms and complaints that have the potential to be associated with silicone breast implants. Today various social media platforms as well as physicians can be found disseminating false, unproven, and largely anecdotal information regarding the safety of these devices and even support unwarranted and possibly morbid procedures such as en bloc capsulectomy. The article that follows highlights the current scientific evidence available on the safety of silicone breast implants as well as the concerns that remain about these devices in light of recent consumer concern and social media reports about the possible existence of a “silicone breast implant illness” syndrome.
Breast implants and cancer risk
In 1995, a case series was published detailing three women with silicone breast implants who were diagnosed with cutaneous T-cell lymphoma (16,17). Such reports were the impetus that sparked the concern for the potential carcinogenicity of silicone breast implants (16,17). Since that time, many European and North American studies have been conducted in order to further investigate the possible association between cancer and these devices (12,18-34) (Table 1).
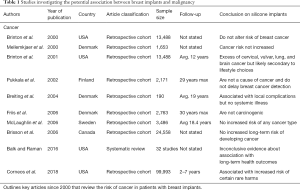
Full table
Breast cancer
The incidence of breast cancer in patients with silicone implants has been well studied and the extensive literature that exists confirms that there is no association between these devices and breast cancer (12,19-31,33-39). However, because of their location, their use in oncologic reconstruction, and their potential to adversely affect breast mammography, the risk of breast cancer development in these patients is of utmost concern.
After the IOM suggested that breast implants may affect the accuracy of routine mammographic breast cancer screening (40), multiple case reports were published that hypothesized that opaque breast implants may interfere with mammographic breast visualization as well as breast physical exam (41-44). This in turn would delay breast cancer diagnosis and result in worse prognosis for those affected. These reports were fundamentally flawed, considering that most included many women who underwent screening mammography without utilization of the Eklund implant displacement technique (45). While the sensitivity of screening mammography may be reduced by the presence of breast implants, there is clear scientific evidence to support that this patient population does not present with more advanced stages of breast cancer or suffer from lower survival rates following diagnosis (19,21,46-50).
The International Agency for Research on Cancer’s (IARC) 1999 report reaffirmed that there is a strong lack of evidence supporting silicone breast implants as carcinogenic to native breast tissue (36). Such conclusions were supported by the IOM Committee on the Safety of Silicone Breast Implants (40). In a meta-analysis, Noels and colleagues failed to identify any association between silicone breast implants and an increased breast cancer incidence; recent publications have confirmed these conclusions (23,51) (Figure 1).
Other malignancies
While reports have linked silicone breast implants to malignancies including brain, cervical, vulvar, lung, in addition to non-melanoma skin cancer, the data does not support these findings (4,12,13,52,53). At the turn of the 20th century, multiple independent scientific review boards formed to discuss the current safety of silicone breast implants and all supported that silicone breast implants had no increased incidence of cancer (13,35-37,40). Since that time, the literature has been updated with many studies conducted to better quantify the risk of cancer in women with breast implants (12,19-23,28,29,34,38,39). Of these published reports, many reached conclusions that supported a similar incidence of cancer in these women compared to that of the general population (19-22). However, in 2018, the largest study of patient safety and implant-specific outcomes for breast implants found that patients with Mentor Corp. silicone implants were 1.54 (95% confidence interval (CI), 1.42–1.68) times more likely to develop a cancer diagnosis compared to the general population (12).
Brinton et al. reported a slight excess of cancer in patients with breast implants. Although the study demonstrated elevated risk for cervical, vulvar, brain cancer, and leukemia, comparison to women who had undergone other types of plastic surgery showed no difference. As with many of these studies, the author’s discussion is of key value as they explain that such observed differences were likely due to selection bias as well as unvalidated cancer diagnoses (29). In evaluating these health concerns, one must recognize that the literature has consistently demonstrated that women with breast implants have different patient demographics as well as lifestyle and/or reproductive characteristics compared to the general population (54-57).
To date there have been multiple large-scale incidence studies examining the risk of brain cancer in patients with silicone breast implants (12,19-22,58), as well as 5 mortality studies (38,59-61). Consistently, these studies support no correlation between silicone breast implants and either an increased incidence of brain cancer or increased mortality from brain cancer in this patient population.
In 2007, McLaughlin et al.’s original paper definitively reached the conclusion that silicone breast implants have no causal relationship regarding incidence of newly diagnosed breast cancer. Today, much of the existing literature continues to support this conclusion. A recent multicenter 2017 observational study examined the long-term safety of women with Natrelle silicone breast implants. The 55,279 patients enrolled in this study represented an interim data set that was subsequently reported in full by Coroneos et al. in 2018 (12). The 2017 study found that silicone breast implants were associated with no increased risk for any cancer diagnosis (62). And while newly published data found increased incidence of melanoma in patients with Mentor Corp breast implants, the authors recognize that this is largely due to patient behavior rather than a direct consequence of silicone breast implants (12). Women with breast implants are more likely to have increased UV light exposure secondary to sunbathing or outdoor lifestyles. Plastic surgeons must council these patients as to the negative effects of excessive UV exposure and emphasize the importance of UV protection while outside.
Recently a well-documented link between textured silicone breast implants and a rare form of anaplastic large cell lymphoma (ALCL) has been reported. Its incidence ranges from one in 3,800 and one in 30,000 cases per 100,000 women with breast prostheses per year. Despite this association the US Food and Drug administration continues to endorse silicone breast implants as safe devices and that this rare form of disease is specifically linked to textured devices (51) (Figure 2).
Breast implants and connective tissue disease (CTD)
The FDA defines CTD as any disorder that affects the connective tissue of the body either through genetic inheritance (fibromyalgia), autoimmune dysfunction (rheumatoid arthritis), or other types of exposure as is the case with scurvy (4). Additional examples of these conditions include scleroderma, Sjögren’s syndrome, and systemic lupus erythematosus (SLE), in addition to many others. Because the incidence and prevalence of these conditions are quite low, fibromyalgia 1,128 per 100,000 women and scleroderma 3 per 100,000 women per year, a very large study of sufficient duration is required to conclude a causal relationship between breast implants and these diseases (63,64).
CTD and silicone implants
It has long been thought that silicone breast implants may place patients at risk for the development of CTD by exposing patients to unsafe levels of systemic silicone. Prior to 2007, the literature contained two highly powered studies which both supported that silicone breast implant rupture carried no increased risk of CTD development (65,66). On the contrary, one prior study found that women with isolated extracapsular implant rupture had an increased incidence of self-reported Raynaud syndrome [odds ratio (OR) =4.2; 95% CI, 1.1–16.0] and “other CTD” (OR =2.7; 95% CI, 0.8–8.5) but failed to discern whether or not the onset of these symptoms was before or after receiving their silicone breast implants (67).
The evidence on breast implant rupture is inconclusive and prior to 2004, all but one published study reached conclusions which supported no association between silicone breast implants and increased incidences of CTD (40,68-75). At that time, one study published in 1996 which examined a large cohort of female health workers demonstrated a relationship between silicone breast implants and CTD (76). The study reported a relative risk (RR) of 1.24 (95% CI, 1.08–1.41) for any self-reported combined CTD in women with silicone breast implants. It is key to recognize that of these self-reported diagnoses, only 22.7% could be found in patient’s individual medical records. Further analysis of the relationship between these devices and specific CTDs failed to report statistically significant increases in disease incidence (77). This was not the only study found to be subject to over reporting and diagnostic biases, as this was also evident in a US cohort study that looked at CTD in 7,234 women in the US with breast implants (78). Upon closer examination of individuals’ health records by rheumatologists, only a small percent of these self-reported cases of CTD were deemed as “likely”.
A large population-based Danish study carefully examined the health records of women with silicone breast implants and compared these to those who had undergone breast reduction surgery (79). Long-term follow-up of 13.4 years revealed that women with silicone breast implants had no significant increase in the incidence of any specific CTD or any of the CTDs combined. Brinton et al. included a category of conditions termed “other disorders” for which they reported a risk ratio of 1.4 (95% CI, 0.8–2.6) prior to 1992 and 3.6 (95% CI, 1.9–7.0) for the period that followed (78). The self-reported data found in the study above and was published in a time marked by widespread litigation and was likely ridden with inaccurate and biased reporting study subjects. Of note, it is important when reviewing these studies to recognize that there is no available saline implant control group which would allow for more accurate and definitive comparisons.
The 1999 IOM40 report found no “convincing evidence for atypical connective tissue or rheumatic disease or a novel constellation of signs and symptoms in women with silicone breast implants”. In response to this report, the US Federal Court-appointed National Science Panel requested a systematic review of the current literature be conducted. Tugwell et al.’s review was utilized to assist in analyzing testimony as it was presented in various lawsuits against breast implant manufacturers (74). Like other prior reports, its conclusion supported the safety of silicone breast implants and did not link these devices to increased risk of CTD thereby discrediting much of the expert testimony that had been brought against the defendants.
In May 2011, Lipworth et al., who of note were paid consultants of implant manufacturers, published a paper entitled “Silicone breast implants and connective tissue disease: no association” (80). As much uncertainty remained regarding the long-term safety of these devices, the purpose of this paper was to assist in reassuring those who still seemed skeptical about the conclusions reached in prior studies. The article ultimately concluded that the health concerns being broadcast to the general public regarding the safety of silicone breast implants was a result of “unprecedented large-scale product liability litigation” rather than sound scientific evidence. The editorial references 18 large-scale cohort studies, 11 case-control studies, and 13 additional independent meta-analyses and critical reviews in order to support their conclusion. The reported results showed a slightly increased risk of self-reported CTDs in women with breast implants (RR 1.24; 95% CI, 1.08–1.41). The RR for each individual CTD showed no statistical significance despite demonstrating minor elevations compared to the general public. Of note many of these reported diagnoses were later unable to be confirmed through medical record review (77).
A National Science Panel Report published in 1998 (81), the 1999 IOM report, and a 2011 FDA review all supported that there was no evidence to link silicone breast implants with an increased incidence of CTD (4,40). It is important to recognize that the authors of these reports all acknowledged that further studies were warranted given limitations to the existing evidence. In 2018, Coroneos et al. published the largest comprehensive study of long-term patient safety in those with silicone breast implants in The Annals of Surgery (12). This prospective analysis of nearly 100,000 patients with 7-year follow reported on multiple CTDs that showed incidences that were greater than double that of the general population. Such conclusions clearly contradicted the interim analysis of the same dataset that was discussed previously. These included Mentor Corp. patients with Sjögren’s syndrome (SIR: 8.14, 95% CI, 6.24–10.44), scleroderma (SIR: 7.00, 95% CI, 5.12–9.34), and rheumatoid arthritis (SIR: 5.96; 95% CI, 5.35–6.62). Additionally, it showed an increased risk of developing multiple sclerosis and myositis, although both at rates less than twice that of the general population. The data presented for patients with Allergan Inc. silicone breast implants was significantly stronger as 7-year follow-up data was based on physician confirmed medical diagnoses and the follow-up rate was significantly higher than for that of Mentor Corp. patients. Patients that underwent revision of prior breast reconstruction with Allergan Inc. implants had incidence ratios greater than 2.0 for scleroderma, Sjögren’s syndrome, and both dermatomyositis and polymyositis at 7-year follow up. Finally, Coroneos et al. reported 500 autoimmune events in the silicone implant cohort compared to five events in those with saline devices. The authors of this report acknowledge the shortcomings of their study, and urge plastic surgeons that although Mentor Corp. data was based on patient reported diagnoses, plastic surgeons and all physicians must be attune to the fact that when patients present to their offices with such complaints, they must not be ignored and referred for evaluation by appropriate medical specialists.
Of note, 1 year prior in 2017, a study was published that represented an interim analysis of the same dataset reported by Coroneos et al. 55,279 women over a 5-year follow-up period were included (12). The analysis concluded that women with silicone gel-filled implants had no increased risk of any CTD. This study was published 4 months after the final data became publicly available.
Results of the 2018 study by Coroneos et al. are not isolated findings (12). The largest meta-analysis to date written by Balk et al. which pooled outcomes from 32 observational studies as well as a recent review article published in 2018 found statistically significant increased incidence of autoimmune/rheumatic disorders, Sjögren’s syndrome, systemic sclerosis, and sarcoidosis in women with silicone breast implants (23,82).
As expected, a media craze ensued following the release of Coroneos et al.’s 2018 article. At this time the FDA released a statement urging both the public and health care professionals to view their conclusions with caution as the study has major shortcomings. The statement addresses that while the analysis of the data was meticulous and well conducted, the process used for data collection was designed by the implant manufacturers and not without inconsistency and bias. Critics of the 2018 article must recognize that these shortcomings were directly addressed by the authors of the study as well as in an editorial response by Colwell et al. (83). Binita Ashar, MD reminds readers of a sentiment shared by most evaluating this long-term health concern which is that the current evidence “does not conclusively demonstrate an association” and that “more evaluation is required” (84). Colwell et al.’s critique also highlights that the authors analyze a much smaller group of patients (<34,000 vs. 99,993) for 7 years despite previous data concerns of poor follow-up and issues with data acquisition. Readers must understand that in addition to poor follow-up, a large subset of data was patient reported and had no physician confirmation. It is not surprising that this patient reported data provided by Mentor ultimately had the greatest association with rare adverse health events; associations which were not upheld through the analysis of 25,219 Allergan patients. Outcomes were compared to “normative” populations and thus failed to control for many confounding variables. These methodological flaws prohibit any definitive conclusions to be reached from this article.
In summary, recent data suggests that although originally refuted, breast implants may have an association with certain specific CTD, a fact which must be explicitly communicated to patients interested in pursuing breast reconstruction or augmentation with silicone breast implants. Governing bodies such as the IOM and FDA strongly reaffirm that breast implants are overwhelmingly safe devices with a low overall risk of developing such conditions (Figure 3).
Breast implants and mental health
Plastic surgery is a unique surgical specialty in that many of these procedures can have a profound effect on patients’ psychiatric wellbeing. It is widely acknowledged that women with breast implants report higher rates of psychotropic medication use including both antidepressants and anxiolytics (18). Like other studies discussed above, one must recognize that often such symptoms of depression are likely present prior to breast augmentation and that perhaps certain individual’s mental health will not benefit from such devices secondary to either their prior diagnosis or predisposition to such conditions.
Previous literature has found an association between elevated rates of suicide and breast implants in the US (59-61,85-87). These conclusions were refuted in Singh et al.’s. 2017 publication which stated that the suicide rate (10.6 events per 100,000 person-years) was not significantly higher than that of the national norm (62). Important to recognize is that those studies who concluded an association with increased suicide rates did not account for or disclose if those patients suffered from a higher incidence of prior underlying psychopathology including but not limited to depression and or anxiety (88). One study conducted in Denmark showed that women undergoing breast augmentation had higher rates of hospitalization for psychiatric illness compared to both women who had undergone breast reduction or other cosmetic surgery (60) (Figure 4, Table 2).
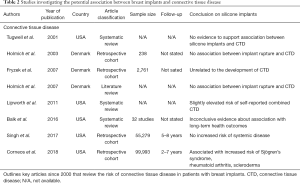
Full table
Breast implants and neurologic disease
Multiple case reports published in the early 1990’s highlighted women with silicone breast implants who developed various neurologic conditions including multiple sclerosis, “multiple sclerosis type syndrome”, various peripheral neuropathies, and “atypical neurological disease syndrome”. Three well powered cohort studies were subsequently published in response in order to better evaluate the association between silicone breast implants and various neurologic conditions (89-91). The authors of these aforementioned studies failed to conclude an association between silicone breast implants and this wide array of neurologic disease (13). As a result, the American Academy of Neurology published a statement which explained that claims made in prior case reports were insufficient to establish a causal relationship due to the methodologically unsound nature of these reports (92). In conducting this review no new evidence has been reported since McLaughlin reached this same conclusion in his 2007 article (Figure 5).
Pregnancy concerns and breast implants
In the mid-1990s multiple case reports were published describing children born to women with silicone breast implants who suffered from various adverse health conditions including irritability, fatigue, and difficulty swallowing (93-98). As isolated case reports, these Level V studies contained obvious selection biases as many of the children exemplified in these reports were born to parents whose families had a strong history of scleroderma and esophageal dysmotility.
Despite four large epidemiologic studies, there is no evidence to suggest a causal relationship between poor neonatal health outcomes and silicone breast implants. The first of these articles by Kjøller et al. found that children born to women with breast implants demonstrated a higher than expected incidence of esophageal disorders (99). However, these same outcomes were seen in children born to women who had undergone breast reduction surgery as well as in children born prior to their mother’s breast augmentation surgery. In a follow-up study, they observed higher than expected rates of esophageal disorders for children born before (O/E =2.0; 95% CI, 1.3–2.8) but not after (O/E =1.3; 95% CI, 0.5–2.9) maternal breast implant surgery with similar excess seen both before (O/E =2.1; 95% CI, 0.5–2.9) and after (O/E =1.6; 95% CI, 1.1–2.3) breast reduction surgery (100). No excess of rheumatic disease was seen. Like the first study, adverse health outcomes were unrelated to silicone breast implants as they were seen in children born both before and after a mother’s breast implant surgery as well as in mothers who underwent breast reduction. A third study from Sweden that looked at 5,874 children born to women with breast implants supported the findings stated above (101). The fourth and final study conducted in Finland by Hemminki et al. was of little utility as it was ridden with obvious design flaws including lack of an adequate control in addition to multiple confounding variables (102).
A common concern of women with breast implants is how these devices affect their ability to breast feed. The data shows that 79.4% of women with silicone breast implants are able to breast feed at least one child with the most common complication being insufficient milk production in 20% of cases, a number which closely mirrors that of the general population (103).
Educating patients seeking breast implant removal
The choice to remove one’s breast implants has always been solely the choice of the patient. Afterall, the decision to undergo surgery was purely elective. It is therefore the responsibility of the plastic surgeon to appropriately council patients on the risks and benefits of breast implant explantation as well as the physical defect often left following breast explantation surgery. Implant removals are not all created equal. Patients must know that there are a variety of degrees of capsulectomy/capsulotomy currently performed today. Defining the terminology is critical, since confusion exists even among plastic surgeons:
- En bloc explantation—surgeon leaves the capsule tissue intact on the breast implant and removes the capsule and implant as one unit. Most surgeons prefer to perform en bloc implant removal through an inframammary fold incision since visualization is challenging through a periareolar or transaxillary approach. Increasingly, patients desiring explanation are requesting en bloc resection since they believe it leads to less “contamination”. However, many surgeons prefer to perform an anterior capsulectomy with the implant and leave the base of the capsule intact to avoid potential complications of posterior capsulectomy (i.e., pneumothorax, chest wall injury) especially if the patient has very thin chest wall musculature.
- Explant with total capsulectomy—surgeon removes the implants and then removes all capsule tissue. This is not done en bloc (i.e., as one piece) necessarily.
- Explant with partial capsulectomy—surgeon removes the implants and then removes a portion of the capsule tissue.
- Open capsulotomy—capsule or scar tissue surrounding the implant is released surgically and left in the patient. Typically, this is done by scoring the tissue with electrocautery. In cases of capsular contracture, an acellular dermal matrix material may be used as a spacer to prevent recurrent capsular contracture.
In cases of ALCL patients require an en bloc removal of the breast implant and surrounding capsule on all sides as one piece. En bloc implant and capsule removal requires a skilled plastic surgeon with experience as removal of the capsule off the chest wall has inherent risks. Particularly for patients who have capsules that are adherent to their chest wall, great care must be taken to avoid inadvertent injury to underlying chest wall structures; as this may lead to pneumothorax.
Plastic surgeons have previously examined the clinical outcomes in patients who undergo explantation for symptoms of physician confirmed diagnosis of rheumatic disease and other autoimmune conditions (104). Implant explanation has continued to become an area of focus as patients have growing concerns regarding potential adverse health effects of silicone breast implants.
Management of the post-explant breast
While patients seeking removal of their breast implants may be focused on explantation, plastic surgeons must address both explantation and secondary revision surgery at the time of consultation. In light of this, it is imperative to understand how both time and the breast implant cause structural changes to the breast footprint and overlying soft tissue. The expansive force of the implant can leave a residual deformity mirroring a post-mastectomy patient depending on the patient’s pre-augmentation breast characteristics and augmentation size. Contrary to popular belief, the breast implant does not only affect the overlying glandular tissue and breast skin but also affects the pectoralis muscle both in submuscular and subglandular breast augmentation.
The first step to any successful explantation and revision reconstruction is detailed patient education. Patient’s must be part of the decision-making process and plastic surgeons must address all of these complex issues is an easy to understand fashion. Once this is done it is up to the plastic surgeon to perform a detailed patient assessment to allow for proper operative planning and decision making.
Secondary breast revision must focus on two key components: (I) soft tissue contouring and (II) volume restoration. Breast contouring must focus on reshaping the breast mound repositioning the nipple-areola complex. Degree of preoperative ptosis, amount of nipple elevation needed, diameter of areola, and thickness of native breast parenchyma all factor into this decision-making process. In an article by Rohrich et al., the authors utilize three main criteria to determine if staging these procedures is necessary: (I) smoking status, (II) nipple elevation >4 cm, (III) breast parenchyma thickness <4 cm (105).
Volume restoration becomes a difficult problem to fix as some patients are not interested in implant exchange despite substitution of implant surface or fill characteristics. In light of this, autologous fat grafting for revision breast augmentation is seen as a viable and reliable option, a trend which has been gaining much popularity in recent years. Ideally it is performed at the time of explantation but it too can be done in a delayed fashion. Fat grafting is not recommended prior to mastopexy.
Conclusions
The purpose of this review article is to discuss the current state of scientific evidence related to the safety of silicone breast implants. In times of uncertainty, unwanted noise can easily distort research-based evidence. It is the responsibility of all physicians, especially plastic surgeons, to always put patient safety first and to critically self-evaluate our practices and the industry partners who serve our patients. Few medical devices have undergone the degree of scrutiny and speculation as silicone breast implants.
At the present state, there is overwhelming evidence to support the safety of silicone breast implants, a fact which is echoed in the FDA’s updated position on the use of silicone breast implants. Ultimately the decision to obtain, keep, or remove breast implants is the choice of the patient; something that surgeons have a responsibility to uphold and support. If a patient chooses to remove their breast implants it is important to find a board-certified plastic surgeon with expertise in breast surgery. It is then the job of the plastic surgeon to support their patient’s decision by providing sound medical advice which includes presenting patients with facts regarding health risks associated with silicone breast implants. The patient must come first, it is their body and therefore their ultimate decision as to what is best for them as breast implantation is an elective medical decision. If a patient chooses to have their implants removed they should consider having the entire capsule removed, unless the posterior capsule is adherent to the chest wall which may increase the risk of pneumothorax. In cases of ALCL or ruptured implants with thick calcified capsule, a total capsulectomy is mandated (106).
To the best of our body of scientific knowledge to date, there have not been any concrete or evidence-based studies or peer reviewed data concerning the formation of a new syndrome “breast implant illness”.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Charles E. Butler, Carrie Chu, and Margaret Roubaud) for the series “New Frontiers in Breast Reconstruction” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-231). The series “New Frontiers in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. Dr. RR reports personal fees from Eriem Surgical, Inc., personal fees from Thieme Medical Publishing, grants from Allergan, Inc., grants from Galderma, grants from MTF Biologics, other from Merz North America, other from Medical Seminars of Texas, LLC., outside the submitted work. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cosmetic Surgery National Data Bank Statistics. Aesthet Surg J 2017;37:1-29.
- Calobrace MB, Capizzi PJ. The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg 2014;134:6S-11S. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017;6:148-53. [Crossref] [PubMed]
- FDA. FDA Update on the Safety of Silicone Gel-Filled Breast Implants Center for Devices and Radiological Health. Center for Devices and Radiological Health U.S. Food and Drug Administration, June 2011.
- Nanayakkara PW, de Blok CJ. Silicone Gel Breast Implants: What We Know About Safety After All These Years. Ann Intern Med 2016;164:199-200. [Crossref] [PubMed]
- Stevens WG, Calobrace MB, Alizadeh K, et al. Ten-year Core Study Data for Sientra's Food and Drug Administration-Approved Round and Shaped Breast Implants with Cohesive Silicone Gel. Plast Reconstr Surg 2018;141:7S-19S. [Crossref] [PubMed]
- Hammond DC, Canady JW, Love TR, et al. Mentor Contour Profile Gel Implants: Clinical Outcomes at 10 Years. Plast Reconstr Surg 2017;140:1142-50. [Crossref] [PubMed]
- Spear SL, Murphy DK, Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133:1354-61. [Crossref] [PubMed]
- Stevens WG, Harrington J, Alizadeh K, et al. Eight-year follow-up data from the U.S. clinical trial for Sientra's FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J 2015;35 Suppl 1:S3-10. [Crossref] [PubMed]
- Cunningham B, McCue J. Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. Erratum in: Aesthetic Plast Surg 2009;33:439. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014;134:12S-7S. [Crossref] [PubMed]
- Coroneos CJ, Selber JC, Offodile AC 2nd, et al. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann Surg 2019;269:30-6. [Crossref] [PubMed]
- McLaughlin JK, Lipworth L, Murphy DK, et al. The safety of silicone gel-filled breast implants: a review of the epidemiologic evidence. Ann Plast Surg 2007;59:569-80. [Crossref] [PubMed]
- Eaves FF 3rd, Rohrich RJ, Sykes JM. Taking evidence-based plastic surgery to the next level: report of the second summit on evidence-based plastic surgery. JAMA Facial Plast Surg 2013;15:314-20. [Crossref] [PubMed]
- Thoma A, Kaur MN, Hong CJ, et al. Methodological guide to adopting new aesthetic surgical innovations. Aesthet Surg J 2015;35:308-18. [Crossref] [PubMed]
- Duvic M, Moore D, Menter A, et al. Cutaneous T-cell lymphoma in association with silicone breast implants. J Am Acad Dermatol 1995;32:939-42. [Crossref] [PubMed]
- Gidengil CA, Predmore Z, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg 2015;135:713-20. [Crossref] [PubMed]
- Breiting VB, Hölmich LR, Brandt B, et al. Long-term health status of Danish women with silicone breast implants. Plast Reconstr Surg 2004;114:217-26; discussion 227-8. [Crossref] [PubMed]
- Friis S, Hölmich LR, McLaughlin JK, et al. Cancer risk among Danish women with cosmetic breast implants. Int J Cancer 2006;118:998-1003. [Crossref] [PubMed]
- Pukkala E, Boice JD Jr, Hovi SL, et al. Incidence of breast and other cancers among Finnish women with cosmetic breast implants, 1970-1999. J Long Term Eff Med Implants 2002;12:271-9. [Crossref] [PubMed]
- McLaughlin JK, Lipworth L, Fryzek JP, et al. Long-term cancer risk among Swedish women with cosmetic breast implants: an update of a nationwide study. J Natl Cancer Inst 2006;98:557-60. [Crossref] [PubMed]
- Brisson J, Holowaty EJ, Villeneuve PJ, et al. Cancer incidence in a cohort of Ontario and Quebec women having bilateral breast augmentation. Int J Cancer 2006;118:2854-62. [Crossref] [PubMed]
- Balk EM, Earley A, Avendano EA, et al. Long-Term Health Outcomes in Women With Silicone Gel Breast Implants: A Systematic Review. Ann Intern Med 2016;164:164-75. [Crossref] [PubMed]
- Malone KE, Stanford JL, Daling JR, et al. Implants and breast cancer. Lancet 1992;339:1365. [Crossref] [PubMed]
- Park AJ, Black RJ, Sarhadi NS, et al. Silicone gel-filled breast implants and connective tissue diseases. Plast Reconstr Surg 1998;101:261-8. [Crossref] [PubMed]
- McLaughlin JK, Nyren O, Blot WJ. Cancer Risk Among Women with Cosmet Breast Implants: a Population-Based cohort Study in Sweden. J Natl Cancer Inst 1998;90:156-8. [Crossref] [PubMed]
- Brinton LA, Malone KE, Coates RJ, et al. Breast Enlargement and Reduction: Results from a Breast Cancer Case-Control Study. Plast Reconstr Surg 1996;97:269-75. [Crossref] [PubMed]
- Brinton LA, Lubin JH, Burich MC, et al. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 2000;11:819-27. [Crossref] [PubMed]
- Brinton LA, Lubin JH, Burich MC, et al. Cancer risk at sites other than the breast following augmentation mammoplasty. Ann Epidemiol 2001;11:248-56. [Crossref] [PubMed]
- Bryant H, Brasher P. Breast implants and breast cancer--reanalysis of a linkage study. N Engl J Med 1995;332:1535-9. [Crossref] [PubMed]
- Deapen DM, Bernstein L, Brody GS. Are breast implants anticarcinogenic? A 14-year follow-up of the Los Angeles Study. Plast Reconstr Surg 1997;99:1346-53. [Crossref] [PubMed]
- Gabriel SE, O'Fallon WM, Kurland LT, et al. Risk of connective-tissue diseases and other disorders after breast implantation. N Engl J Med 1994;330:1697-702. [Crossref] [PubMed]
- Kern KA, Flannery JT, Kuehn PG. Carcinogenic potential of silicone breast implants: a Connecticut statewide study. Plast Reconstr Surg 1997;100:737-47; discussion 748-9. [Crossref] [PubMed]
- Mellemkjaer L, Kjøller K, Friis S, et al. Cancer occurrence after cosmetic breast implantation in Denmark. Int J Cancer 2000;88:301-6. [Crossref] [PubMed]
- European Committee on Quality Assurance and Medical Devices in Plastic Surgery. Consensus Declaration on Breast Implants, 23-6-2000. Israel, European Committee on Quality Assurance (EQUAM). 4th Consensus Declaration.
- World Health Organization. International Agency for Research on Cancer. Surgical Implants and Other Foreign Bodies: IARC Monograph on the Evaluation of Carcinogenic Risks to Humans. Vol 74.; 1999.
- National Institutes of Health. Breast implants: status of research at the National Institutes of Health. Amended report 3/24/2005.
- Lipworth L, Tarone RE, Friis S, et al. Cancer among Scandinavian women with cosmetic breast implants: a pooled long-term follow-up study. Int J Cancer 2009;124:490-3. [Crossref] [PubMed]
- Noels EC, Lapid O, Lindeman JH, et al. Breast implants and the risk of breast cancer: a meta-analysis of cohort studies. Aesthet Surg J 2015;35:55-62. [Crossref] [PubMed]
- Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants. Safety of Silicone Breast Implants. Bondurant S, Ernster V, Herdman R. editors. Washington (DC): National Academies Press (US); 1999.
- Fajardo LL, Harvey JA, McAleese KA, et al. Breast cancer diagnosis in women with subglandular silicone gel-filled augmentation implants. Radiology 1995;194:859-62. [Crossref] [PubMed]
- Silverstein MJ, Handel N, Gamagami P, et al. Breast cancer in women after augmentation mammoplasty. Arch Surg 1988;123:681-5. [Crossref] [PubMed]
- Silverstein MJ, Handel N, Gamagami P, et al. Breast cancer diagnosis and prognosis in women following augmentation with silicone gel-filled prostheses. Eur J Cancer 1992;28:635-40. [Crossref] [PubMed]
- Silverstein MJ, Gierson ED, Gamagami P, et al. Breast cancer diagnosis and prognosis in women augmented with silicone gel-filled implants. Cancer 1990;66:97-101. [Crossref] [PubMed]
- Eklund GW, Busby RC, Miller SH, et al. Improved imaging of the augmented breast. AJR Am J Roentgenol 1988;151:469-73. [Crossref] [PubMed]
- Deapen D, Hamilton A, Bernstein L, et al. Breast cancer stage at diagnosis and survival among patients with prior breast implants. Plast Reconstr Surg 2000;105:535-40. [Crossref] [PubMed]
- Hoshaw SJ, Klein PJ, Clark BD, et al. Breast implants and cancer: causation, delayed detection, and survival. Plast Reconstr Surg 2001;107:1393-407. [Crossref] [PubMed]
- Miglioretti DL, Rutter CM, Geller BM, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA 2004;291:442-50. [Crossref] [PubMed]
- Tang SS, Gui GP. A review of the oncologic and surgical management of breast cancer in the augmented breast: diagnostic, surgical and surveillance challenges. Ann Surg Oncol 2011;18:2173-81. [Crossref] [PubMed]
- Kam K, Lee E, Pairawan S, et al. The Effect of Breast Implants on Mammogram Outcomes. Am Surg 2015;81:1053-6. [Crossref] [PubMed]
- Rohrich RJ, Kaplan J, Dayan E. Silicone Implant Illness: Science versus Myth? Plast Reconstr Surg 2019;144:98-109. [Crossref] [PubMed]
- Deapen DM, Hirsch EM, Brody GS. Cancer risk among Los Angeles women with cosmetic breast implants. Plast Reconstr Surg 2007;119:1987-92. [Crossref] [PubMed]
- Brinton LA. The relationship of silicone breast implants and cancer at other sites. Plast Reconstr Surg 2007;120:94S-102S. [Crossref] [PubMed]
- Kjøller K, Hölmich LR, Fryzek JP, et al. Characteristics of women with cosmetic breast implants compared with women with other types of cosmetic surgery and population-based controls in Denmark. Ann Plast Surg 2003;50:6-12. [Crossref] [PubMed]
- Cook LS, Daling JR, Voigt LF, et al. Characteristics of women with and without breast augmentation. JAMA 1997;277:1612-7. [Crossref] [PubMed]
- Brinton LA, Brown SL, Colton T, et al. Characteristics of a population of women with breast implants compared with women seeking other types of plastic surgery. Plast Reconstr Surg 2000;105:919-27; discussion 928-9. [Crossref] [PubMed]
- Fryzek JP, Weiderpass E, Signorello LB, et al. Characteristics of women with cosmetic breast augmentation surgery compared with breast reduction surgery patients and women in the general population of Sweden. Ann Plast Surg 2000;45:349-56. [Crossref] [PubMed]
- McLaughlin JK, Lipworth L. Brain cancer and cosmetic breast implants: a review of the epidemiologic evidence. Ann Plast Surg 2004;52:115-7. [Crossref] [PubMed]
- Brinton LA, Lubin JH, Murray MC, et al. Mortality rates among augmentation mammoplasty patients: an update. Epidemiology 2006;17:162-9. [Crossref] [PubMed]
- Jacobsen PH, Hölmich LR, McLaughlin JK, et al. Mortality and suicide among Danish women with cosmetic breast implants. Arch Intern Med 2004;164:2450-5. [Crossref] [PubMed]
- Villeneuve PJ, Holowaty EJ, Brisson J, et al. Mortality among Canadian women with cosmetic breast implants. Am J Epidemiol 2006;164:334-41. [Crossref] [PubMed]
- Singh N, Picha GJ, Hardas B, et al. Five-Year Safety Data for More than 55,000 Subjects following Breast Implantation: Comparison of Rare Adverse Event Rates with Silicone Implants versus National Norms and Saline Implants. Plast Reconstr Surg 2017;140:666-79. [Crossref] [PubMed]
- Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol 2006;12:124-8. [Crossref] [PubMed]
- Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003;48:2246-55. [Crossref] [PubMed]
- Hölmich LR, Kjøller K, Fryzek JP, et al. Self-reported diseases and symptoms by rupture status among unselected Danish women with cosmetic silicone breast implants. Plast Reconstr Surg 2003;111:723-34. [Crossref] [PubMed]
- Hölmich LR, Lipworth L, McLaughlin JK, et al. Breast implant rupture and connective tissue disease: a review of the literature. Plast Reconstr Surg 2007;120:62S-69S. [Crossref] [PubMed]
- Brown SL, Pennello G, Berg WA, et al. Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women. J Rheumatol 2001;28:996-1003. [PubMed]
- Lipworth L, Tarone RE, McLaughlin JK. Silicone breast implants and connective tissue disease: an updated review of the epidemiologic evidence. Ann Plast Surg 2004;52:598-601. [Crossref] [PubMed]
- Blackburn WD Jr, Everson MP. Silicone-associated rheumatic disease: an unsupported myth. Plast Reconstr Surg 1997;99:1362-7. [Crossref] [PubMed]
- Downing D. Silicone Gel Breast Implants: the UK Independent Review Group Report. J Nutr Environ Med 1998;8:317-9. [Crossref]
- Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med 2000;342:781-90. [Crossref] [PubMed]
- Lamm SH. Silicone breast implants, breast cancer and specific connective tissue diseases: a systematic review of the data in the epidemiological literature. Int J Toxicol 1998;17:497-527. [Crossref]
- Silverman BG, Brown SL, Bright RA, et al. Reported complications of silicone gel breast implants: an epidemiologic review. Ann Intern Med 1996;124:744-56. [Crossref] [PubMed]
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001;44:2477-84. [Crossref] [PubMed]
- Wong O. A critical assessment of the relationship between silicone breast implants and connective tissue diseases. Regul Toxicol Pharmacol 1996;23:74-85. [Crossref] [PubMed]
- Hennekens CH, Lee IM, Cook NR, et al. Self-reported breast implants and connective-tissue diseases in female health professionals. A retrospective cohort study. JAMA 1996;275:616-21. Erratum in: JAMA 1998;279:198. [Crossref] [PubMed]
- Karlson EW, Lee IM, Cook NR, et al. Comparison of self-reported diagnosis of connective tissue disease with medical records in female health professionals: the Women's Health Cohort Study. Am J Epidemiol 1999;150:652-60. [Crossref] [PubMed]
- Brinton LA, Buckley LM, Dvorkina O, et al. Risk of connective tissue disorders among breast implant patients. Am J Epidemiol 2004;160:619-27. [Crossref] [PubMed]
- Fryzek JP, Holmich L, McLaughlin JK, et al. A nationwide study of connective tissue disease and other rheumatic conditions among Danish women with long-term cosmetic breast implantation. Ann Epidemiol 2007;17:374-9. [Crossref] [PubMed]
- Lipworth L, Holmich LR, McLaughlin JK. Silicone breast implants and connective tissue disease: no association. Semin Immunopathol 2011;33:287-94. [Crossref] [PubMed]
- Diamond BA, Hulka BS, Kerkvliet NI, Tugwell P. Silicone Breast Implants in Relation to Connective Tissue Diseases and Immunologic Dysfunction: A Report by a National Science Panel to the Honorable Sam C. Pointer Jr. Coordinating Judge for the Federal Breast Implant Multi-District Litigation. 1998. Available online: http://www.fsc.gov/BREIMLIT/md/1926.htm
- Watad A, Rosenberg V, Tiosano S, et al. Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol 2018;47:1846-54. [Crossref] [PubMed]
- Colwell AS, Mehrara B. Editorial: US FDA Breast Implant Postapproval Studies-Long-term Outcomes in 99,993 Patients. Ann Surg 2019;269:39-40. [Crossref] [PubMed]
- Ashar B. Statement from Binita Ashar, M.D., of the FDA’s Center for Devices and Radiological Health on agency’s commitment to studying breast implant safety. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm620589.htm?utm_campaign=09142018_Statement_FDA+Statement+on+agency’s+commitment+to+studying+breast+implant+safety&utm_medium=email&utm_source=Eloqua&elqTrackId=179F06C0C4E20BA8893DDF6B820619AF&
- Lipworth L, Nyren O, Ye W, et al. Excess mortality from suicide and other external causes of death among women with cosmetic breast implants. Ann Plast Surg 2007;59:119-23; discussion 124-5. [Crossref] [PubMed]
- Brinton LA, Lubin JH, Burich MC, et al. Mortality among augmentation mammoplasty patients. Epidemiology 2001;12:321-6. [Crossref] [PubMed]
- Pukkala E, Kulmala I, Hovi SL, et al. Causes of death among Finnish women with cosmetic breast implants, 1971-2001. Ann Plast Surg 2003;51:339-42; discussion 343-4. [Crossref] [PubMed]
- McLaughlin JK, Lipworth L, Tarone RE. Suicide among women with cosmetic breast implants: a review of the epidemiologic evidence. J Long Term Eff Med Implants 2003;13:445-50. [Crossref] [PubMed]
- Nyrén O, McLaughlin JK, Yin L, et al. Breast implants and risk of neurologic disease: a population-based cohort study in Sweden. Neurology 1998;50:956-61. [Crossref] [PubMed]
- Winther JF, Bach FW, Friis S, et al. Neurologic disease among women with breast implants. Neurology 1998;50:951-5. [Crossref] [PubMed]
- Winther JF, Friis S, Bach FW, et al. Neurological disease among women with silicone breast implants in Denmark. Acta Neurol Scand 2001;103:93-6. [Crossref] [PubMed]
- Ferguson JH. Silicone breast implants and neurologic disorders. Report of the Practice Committee of the American Academy of Neurology. Neurology 1997;48:1504-7. [Crossref] [PubMed]
- Levine JJ, Ilowite NT. Sclerodermalike esophageal disease in children breast-fed by mothers with silicone breast implants. JAMA 1994;271:213-6. Erratum in: JAMA 1994;272:770. [Crossref] [PubMed]
- Levine JJ, Trachtman H, Gold DM, et al. Esophageal dysmotility in children breast-fed by mothers with silicone breast implants. Long-term follow-up and response to treatment. Dig Dis Sci 1996;41:1600-3. [Crossref] [PubMed]
- Levine JJ, Lin HC, Rowley M, et al. Lack of autoantibody expression in children born to mothers with silicone breast implants. Pediatrics 1996;97:243-5. [PubMed]
- Teuber SS, Gershwin ME. Autoantibodies and clinical rheumatic complaints in two children of women with silicone gel breast implants. Int Arch Allergy Immunol 1994;103:105-8. [Crossref] [PubMed]
- Levine JJ, Ilowite NT, Pettei MJ, et al. Increased urinary NO3(-) + NO2- and neopterin excretion in children breast fed by mothers with silicone breast implants: evidence for macrophage activation. J Rheumatol 1996;23:1083-7. [PubMed]
- Gedalia A, Cuéllar ML, Espinoza LR. Skin rash and anti-Ro/SS-A antibodies in an infant from a mother with silicone breast implants. Clin Exp Rheumatol 1995;13:521-3. [PubMed]
- Kjøller K, McLaughlin JK, Friis S, et al. Health outcomes in offspring of mothers with breast implants. Pediatrics 1998;102:1112-5. [Crossref] [PubMed]
- Kjøller K, Friis S, Signorello LB, et al. Health outcomes in offspring of Danish mothers with cosmetic breast implants. Ann Plast Surg 2002;48:238-45. [Crossref] [PubMed]
- Signorello LB, Fryzek JP, Blot WJ, et al. Offspring health risk after cosmetic breast implantation in Sweden. Ann Plast Surg 2001;46:279-86. [Crossref] [PubMed]
- Hemminki E, Hovi SL, Sevón T, et al. Births and perinatal health of infants among women who have had silicone breast implantation in Finland, 1967-2000. Acta Obstet Gynecol Scand 2004;83:1135-40. [Crossref] [PubMed]
- Jewell ML, Edwards MC, Murphy DK, et al. Lactation Outcomes in More Than 3500 Women Following Primary Augmentation: 5-Year Data From the Breast Implant Follow-Up Study. Aesthet Surg J 2019;39:875-83. [Crossref] [PubMed]
- Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg 1997;39:9-19. [Crossref] [PubMed]
- Rohrich RJ, Beran SJ, Restifo RJ, et al. Aesthetic management of the breast following explantation: evaluation and mastopexy options. Plast Reconstr Surg 1998;101:827-37. [Crossref] [PubMed]
- Clemens MW, Brody GS, Mahabir RC, et al. How to Diagnose and Treat Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2018;141:586e-599e. [Crossref] [PubMed]







