
Effects of excitation light intensity on parathyroid autofluorescence with a novel near-infrared fluorescence imaging system: two surgical case reports
Introduction
The intraoperative identification and preservation of the parathyroid glands are vital in thyroid and parathyroid surgeries. During thyroidectomy, the careful preservation of the parathyroid glands is important due to temporary or permanent hypocalcemia caused by hypothyroidism. Diseased parathyroid glands need to be accurately identified and removed during surgery because their incomplete excision results in recurrence. Due to their small size and various anatomical positions, difficulties are associated with distinguishing the parathyroid glands from surrounding tissues, such as the lymph nodes and adipose tissue (1).
Surgeons continue to rely on visual assessments during surgery in order to identify and preserve the parathyroid glands, and the success of this approach is largely dependent on a surgeon’s experience (2). Therefore, a simple and reproducible technique to identify the parathyroid glands during surgery is needed.
In 2011, Paras et al. showed that the parathyroid glands possessed a fluorescent property in the near-infrared (NIR) region and emitted NIR light at a peak wavelength of 820 nm when illuminated with light at 785 nm (3). McWade et al. conducted imaging of parathyroid gland autofluorescence during human thyroidectomy for the first time in 2014 (4). Autofluorescence spectroscopy is an effective technique for intraoperatively visualizing the parathyroid glands (5,6).
LIGHTVISION® (Shimazu, Kyoto, Japan) is a single monitor that evaluates NIR as well as visible light images and three-dimensional images superimposed on them in real-time. The xenon light source of the system provides visible light at a wavelength of 780–800 nm and NIR excitation light at a wavelength of 800–850 nm. Previous studies reported that it was possible to confirm indocyanine green (ICG)/NIR fluorescence without turning off the operating room light in evaluations of vascularized lymph node flaps (7) and lymphography for lymphaticovenular anastomosis (8).
We herein demonstrate the effectiveness of the excitation light to autofluorescence in the intraoperative identification of the parathyroid glands using the novel NIR fluorescence imaging system LIGHTVISION®. We also investigated differences in autofluorescence by the histological background.
The following cases were presented in accordance with the CARE guidelines (9). Available at http://dx.doi.org/10.21037/gs-20-386.
Case presentation
Case 1
A 33-year-old female with a mass of approximately 2.0×1.0×3.0 cm in the left thyroid lobe was diagnosed with papillary thyroid carcinoma. She had no symptoms and she had no previous or family history. An ultrasound examination showed a mass of approximately 2 cm in diameter in the left thyroid lobe and the absence of swollen parathyroid glands. Her preoperative serum calcium level was within the normal range. She underwent left hemi-thyroidectomy and cervical lymph node dissection. The left upper parathyroid gland was easily identified without observations of autofluorescence. After lateral mobilization of the left thyroid lobe and exposure of the recurrent laryngeal nerve, LIGHTVISION® was employed to locate the left lower parathyroid gland. Autofluorescence was observed in the parathyroid gland under white light, and became stronger with NIR excitation light. When adipose tissue was removed from the surface of the parathyroid glands, the intensify of autofluorescence increased. Furthermore, autofluorescence in the parathyroid glands was stronger ex vivo than in vivo (Figure 1). Resected tissue was pathologically diagnosed as normal parathyroid glands and implanted into the left sternocleidomastoid muscle. She had no tetany and her postoperative serum Ca2+ level was within the normal range.
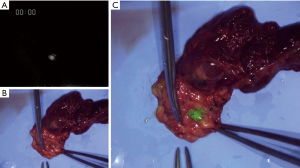
Case 2
A 47-year-old male was referred to our hospital with a high serum Ca2+ level (11.1 mg/dL). He had no symptoms. His sister had been diagnosed with MEN1. Laboratory tests revealed an elevated intact parathyroid hormone (PTH) level (664 pg/mL). Neck ultrasonography showed one enlarged parathyroid gland behind the upper pole of the left thyroid lobe, and 99m-Technetium Methoxy-Isobutyl-Isonitrile (MIBI) scintigraphy revealed uptake in both the upper and lower left thyroid lobes. He was diagnosed with primary hyperparathyroidism due to MEN1 and underwent total parathyroidectomy.
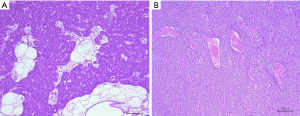
The parathyroid glands were removed during surgery. The left upper gland was the largest, weighing 8,720 mg. Although autofluorescence was weaker than that in Case 1, it was observed in 5 parathyroid glands, but was absent in the left upper gland. To compare the intensity of autofluorescence between the parathyroid glands and surrounding tissues, light was irradiated to the parathyroid glands, thyroid tissues, lymph nodes, adipose tissue, and thymus tissue. Differences in the intensity of autofluorescence were small. When the parathyroid glands were irradiated with a red laser pointer ex vivo, the intensity of autofluorescence significantly increased and the difference in fluorescence intensity from the surrounding tissues was more clearly confirmed (Figure 2A). However, autofluorescence was not observed in the largest gland, even with red laser pointer excitation (Figure 2B). In the postoperative examination, serum Ca2+ and intact PTH levels decreased (serum Ca2+: 7.4 mg/dL and intact PTH: 9 pg/mL). Calcium and vitamin D were replenished, but continued to be replenished one year after surgery. A histological examination revealed the proliferation of chief cells and eosinophilic cells in all glands, diagnosed as hyperplasia (Figure 3). In parathyroid glands other than the upper parathyroid gland, adipose tissue was maintained, but at smaller amounts than the normal parathyroid glands (Figure 3A). The left upper gland contained less adipose tissue and more strongly proliferating chief cells (Figure 3B).
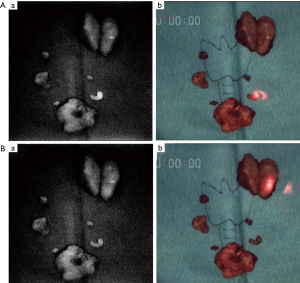
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
In Case 1, autofluorescence was stronger with NIR excitation light. Autofluorescence was weak when adipose tissue was present on the surface of the parathyroid glands. Autofluorescence in the parathyroid glands was stronger ex vivo than in vivo. These results indicated that strong excitation light is needed to increase autofluorescence in the parathyroid glands. A technique that increases the intensity of excitation light to enhance the sensitivity of detecting autofluorescence in the parathyroid glands was considered to be necessary. In Case 2, a red laser pointer was employed to increase the energy of excitation light, and stronger autofluorescence was observed in the parathyroid glands. These results indicated that the light of a red laser pointer has the same wavelength as the excitation light of LIGHTVISION®, and the addition of excitation light increases the visibility of autofluorescence in the parathyroid glands.
In order to increase intraoperative identification rate of the parathyroid glands by autofluorescence, stronger excitation light was considered to be necessary. A limitation of autofluorescence spectroscopy of the parathyroid glands is the difficulty associated with observing autofluorescence in deeply located parathyroid glands because the intensity of fluorescence decreases with increases in the distance from the parathyroid glands to the surface (4,10). As a method to strengthen the excitation light, Ladurner et al. previously reported that a higher gain setting of the camera’s blue channel during NIR autofluorescence imaging may effectively apply a stronger light source; however, this method may cause noise (11). We used a red laser pointer to enhance the excitation light. Our method is very simple, and images of autofluorescence in the parathyroid glands may be visualized without extra gain. However, further studies are needed to confirm the effectiveness of this method because this was a preliminary study.
Autofluorescence was observed in the majority of the parathyroid glands in Case 2, but was weaker than that in Case 1. The largest parathyroid glands showed weak or no autofluorescence even with excitation by the red laser pointer. In the present cases, autofluorescence was weaker in diseased than in normal parathyroid glands. However, Falco et al. reported that parathyroid adenomas showed significantly stronger fluorescence than normal parathyroid glands (12). Furthermore, Ladurner et al. found no significant differences in autofluorescence between parathyroid adenomas, hyperplasia, and normal parathyroid glands (6). The mechanisms underlying autofluorescence in the parathyroid glands currently remain unclear. Furthermore, disease-dependent changes in autofluorescence in these glands have not yet been examined in detail. In the histological examination, the largest gland contained the most strongly proliferating chief cells, while other parathyroid glands showed mild proliferation. The proliferation of parathyroid gland cells may affect the intensity of autofluorescence observed. Therefore, the method described herein may be an effective tool for examining normal parathyroid glands or parathyroid glands with few pathological changes.
Previous studies reported that LIGHTVISION® is an effective tool for evaluating vascularized lymph node flaps (7) and lymphography for lymphaticovenular anastomosis (8). This is the first study to investigate autofluorescence in the parathyroid glands using LIGHTVISION®. One of the advantages of LIGHTVISION® is that autofluorescence in the parathyroid glands may be confirmed without turning off the operation room light. In previous studies, the operation room light needed to be turned off in order to obtain NIR autofluorescence images (4,10). In our method, autofluorescence in the parathyroid glands may be confirmed while the operation room light remains on, which may increase the accuracy of surgical procedures. As another advantage of LIGHTVISION®, NIR and visible light images as well as three-dimensional images superimposed on them may be evaluated on a single monitor in real-time. Kim et al. introduced a technique to visualize the parathyroid glands and surrounding tissues in a single image using a 780-nm collimated light-emitting diode (Thorlabs, Newton, NJ, USA) with an excitation filter appropriate for parathyroid gland autofluorescence, an illuminator (INFRALUX-300, Daekyoung, Korea) for reflection of the entire surgical area, and a digital single-lens (Canon, EOSREBEL T3, Japan) with a camera lens (Canon EF 50 mm f/1.8 II, Japan) including an emission filter (13,14). In our method, the same image may be more easily obtained by switching buttons. This novel imaging system may be an effective tool for the intraoperative identification of the parathyroid glands.
Our method may be useful for the intraoperative identification of normal parathyroid glands during thyroidectomy and slightly enlarged parathyroid glands that cannot be confirmed by ultrasonography or 99m-Technetium MIBI scintigraphy. A red laser pointer may also be an effective tool for easily enhancing excitation light and increasing the detection sensitivity of autofluorescence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-386
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-386). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery 1984;95:14-21. [PubMed]
- Chen H, Wang TS, Yen TW, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg 2010;252:691-5. [PubMed]
- Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014;99:4574-80. [Crossref] [PubMed]
- McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016;159:193-202. [Crossref] [PubMed]
- Ladurner R, Sommerey S, Arabi NA, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017;31:3140-5. [Crossref] [PubMed]
- Tsukuura R, Sakai H, Fuse Y, et al. Novel hands-free near-infrared fluorescence navigation and simultaneous combined imaging for elevation of vascularized lymph node flap. J Surg Oncol 2018;118:588-9. [PubMed]
- Seki Y, Kajikawa A, Yamamoto T, et al. Real-time Indocyanine Green Videolymphography Navigation for Lymphaticovenular Anastomosis. Plast Reconstr Surg Glob Open 2019;7:e2253. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Kim SW, Lee HS, Lee KD. Intraoperative real-time localization of parathyroid gland with near infrared fluorescence imaging. Gland Surg 2017;6:516-24. [Crossref] [PubMed]
- Ladurner R, Lerchenberger M, Al Arabi N, et al. Parathyroid Autofluorescence-How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules 2019;24:2560. [Crossref] [PubMed]
- Falco J, Dip F, Quadri P, et al. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg 2016;223:374-80. [Crossref] [PubMed]
- Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab 2016;101:4646-52. [Crossref] [PubMed]
- Kim SW, Lee HS, Ahn YC, et al. Near-Infrared Autofluorescence Image-Guided Parathyroid Gland Mapping in Thyroidectomy. J Am Coll Surg 2018;226:165-72. [Crossref] [PubMed]


