
Asian and Western practice in thyroid pathology: similarities and differences
Introduction
Western medicine has played a significant role in establishing standardized diagnostic approaches to disease and optimal clinical management of patients. In thyroid pathology most practitioners follow international pathology diagnostic systems developed principally by Western authors and organizations such as the Bethesda system for reporting thyroid fine-needle aspiration (FNA) cytopathology (TBS) (1), the World Health Organization (WHO) classification of tumors of endocrine organs (2), or clinical guidelines published by the Western societies such as the American Thyroid Association (ATA) (3). However, when Western systems are implemented in Asia, Asian pathologists often produce differing results from those seen in the West as shown in several studies (4-11).
The Asian Working Group for Thyroid Cytology/Pathology (Asian WG) was established in 2017 (12) to address the issue of differences between Western and Asian thyroid pathology and cytology practice (13-20). From a practical standpoint, Asian pathologists have to pay close attention to these differences when Western systems are introduced to Asian patient cohorts as Western systems are usually based on data derived from Western patients. This focused series now highlights some of the similarities and differences that are noted when Western systems for thyroid pathology and cytology are implemented in Asian patient cohorts.
What types of differences are there?
This themed issue addresses some of the important differences seen when Western systems for thyroid pathology and cytology are implemented in Asian practice. Some of these are entirely understandable, and are to be expected, whereas others would be regarded as scientifically unacceptable and not appropriate in patient-centered care.
One marked difference is in benign/malignant diagnosis: a disagreement between benign and malignant in a case of non-invasive encapsulated papillary patterned thyroid tumor in a young male patient (21), (Figure 1). See the following link in detail: (https://bit.ly/2FE7hAG). This case was presented at a slide seminar (Thyroid Slide Seminar 2) in the 20th International Congress of Cytology in Sydney in 2019. The majority of the international audience favored a malignant diagnosis, papillary thyroid carcinoma (PTC), whereas some Asian and European pathologists felt that it was a benign tumor, a follicular adenoma (FA) with papillary hyperplasia. The reason for the discrepant diagnosis was most likely the subjective judgement of PTC-like nuclear features; one opinion was that it was malignant PTC in which the nuclear features shown in Figure 1C were considered diagnostic for PTC, but another opinion was that it was a benign FA in which the nuclear features were insufficient for a diagnosis of PTC. Both benign and malignant inter-observer variation was evident when this case was submitted to journals for publication. Although the journal reviewers examined only one still image, the diagnoses varied from benign, borderline, to malignant (Table 1).
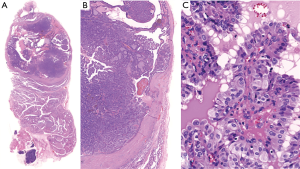

Full table
From these observations together with previous experience of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) (4-6), it is clear that the diagnosis of PTC-like nuclei is subjective and that pathologists have considerable difficulty in distinguishing tumors with PTC-like nuclei from benign lesions (4-6). Thus, the diagnostic distinction of an indolent borderline/precursor tumor (cancer treatment is unnecessary) from a lethal malignancy (cancer treatment is mandatory) can be subjective, and pathologists may be biased if they are uncertain when diagnosing PTC rather than stating that the lesion is diagnostically uncertain as has been pointed out by eminent epidemiologists (22,23). Theoretically, there should be a pre-invasive stage of an invasive RAS mutated PTC with a papillary pattern; the case in Figure 1 was the first reported example, and it was named NEPRAS (non-invasive encapsulated papillary RAS-like thyroid tumor) by Ohba et al. with the aim of preventing unnecessary overtreatment of this lesion as a carcinoma [completion thyroidectomy and radioactive iodine (RAI) ablation] (21). Recently Rosario reported 3 additional patients with NEPRAS and these patients developed no recurrence in 36, 48 and 60 months after surgery (24), which was discussed in an editorial by Jung et al. (25). Further studies are necessary to confirm whether this new borderline tumor entity has a truly favorable outcome or low metastatic potential using a larger patient cohort with extended follow-up data but it highlights some of the diagnostic problems of PTC-type nuclei. An alternative was inclusion criteria for NIFTP to be enlarged and include papillary growth and/or oncocytic features, which could avoid/limit the arbitrary/subjective binary benign/malignant classification of these borderline tumors while at the same time not creating additional borderline entities. Another alternative viewpoint perhaps using WHO classification of tumors of endocrine organs is that this lesion falls within the spectrum of lesions designated as so called ‘well differentiated tumour of uncertain malignant potential’ as it is encapsulated and shows no invasion has molecular features of both follicular carcinoma and also morphology more similar to that seen in classical type PTC (2).
Why is a borderline category necessary for thyroid tumor classification?
Although overtreatment (total thyroidectomy followed by RAI ablation) for equivocal thyroid cancer cases may be necessary for clinicians and patients to prevent under-treatment of malignancies on rare occasions as a safe practice option (1,3,26,27), benign and malignant discrepancies in equivocal cases should be avoided. From a scientific point of view pathologists ought to be able to consistently provide highly reproducible diagnosis of thyroid carcinoma versus benign thyroid lesions but the reality is often quite different (4-6,16,21). Diagnostic discrepancies cause problems in the prognostic assessment of thyroid carcinoma because all existing prognostic data are based on histopathological diagnosis. If benign and malignant disagreements among pathologists are significant, the prognostic data produced by different groups of pathologists is no longer comparable, as demonstrated in the following examples: Figure 1 (encapsulated PTC vs. FA with papillary hyperplasia) (2,21) and the non-invasive encapsulated follicular patterned tumors (follicular variant PTC vs. FA) (4-6,28). The inter-observer variation in diagnosis of PTC-like nuclei as in the RAS mutated thyroid tumor, (NEPRAS papillary pattern) and also in NIFTP (follicular pattern), may explain (I) why there is significant heterogeneity in the positive predictive value of RAS mutations for malignancy in thyroid nodules (29), (II) why the prevalence of BRAFV600E mutation in Asian PTC cohorts was higher than that in Western PTC series (30,31) and (III) why BRAF mutation in Western PTC cohort predicted a poorer prognosis (32) but it was not confirmed in Asian patients (33-35) as Western pathologists and Asian pathologists may have subtly different diagnostic thresholds for papillary carcinoma-type nuclei (please refer to reviews by Dr. Zhu, Dr. Choden, Dr. Nguyen, Dr. Rashid, Dr. Ooi and Dr. Guleria in this focused series) (11). A borderline tumor category has been proposed for thyroid tumor classification by several groups of pathologists (19,28,36-39), which may circumvent some of the problems with this benign/malignant diagnostic dichotomy. A borderline tumor diagnosis by the pathologist instead of the diagnosis of a low-risk carcinoma helps the clinician and patient select more conservative treatment, and thus avoid total thyroidectomy and RAI ablation, which are unnecessary for borderline tumors and low-risk (encapsulated non-invasive) thyroid carcinomas (3). Association of disease terminology and treatment preference was confirmed by a recent survey by Nickel et al. (40).
Encapsulation and absence of invasion
To assess the biological behavior of thyroid tumors, encapsulation and absence of invasion are essential criteria in the diagnosis of borderline/precursor thyroid tumors. Even in invasive thyroid carcinomas, carcinoma cases with encapsulation (but without angioinvasion) have been found to have, in general, more favorable outcomes. Bai et al. from our group found no recurrences in 25 cases of encapsulated PTC in an Asian patient cohort regardless of lymph node metastasis. Piana et al. reported no thyroid cancer deaths in 102 cases of encapsulated invasive thyroid carcinomas, both PTCs and follicular thyroid carcinomas (FTCs), in a European patient series, and Goffredo et al. reported that only 2 patients out of 1,200 with minimally invasive FTCs (encapsulated invasive thyroid carcinoma without PTC-N) died of thyroid cancer in US patient cohorts (41-43). In conclusion, encapsulation, absence of invasion, and absence of BRAFV600E-type PTC nuclear features have become important in the diagnosis of many biologically benign or borderline tumors (2,19,28,36,38,44), for which simple excision is a curative treatment, similar to that for benign lesions. It is predicted that the borderline tumor concept in thyroid tumor classification will expand in the near future (8,36-39,44), as some tumors which are currently classified in the thyroid carcinoma category (minimally invasive FTC, encapsulated conventional type PTC, small localized papillary carcinoma, etc.) have a cancer-specific survival rate of almost 100% at 10 years (41-46) (please see a review by Canberk in this focused series).
Why do we have differences in practice and how should we standardize them?
The differences observed in cohorts of Western and Asian thyroid cancer patients may be caused by multiple factors such as (I) different disease patterns (please refer to reviews by Dr. Choden, Dr. Jung, Dr. Tangnuntachai, Dr. Rashid and Dr. Ngnuyen in this focused series), (II) different practice patterns (please refer to reviews by Dr. Zhu, Dr. Okamoto, Dr. Michael, Dr. Ooi, Dr. Nakra, Dr. Liu and Dr. Guleria in this focused series), (III) diverse epidemiologic backgrounds of patient cohorts (please refer to reviews by Dr. Odate, Dr. Rashid, Dr. Li and Dr. Bai), (IV) variable medical resources (please refer to reviews by Dr. Okamoto, Dr. Michael, Dr. Choden and Dr. Ohori), (V) variable health insurance systems (please refer to a review by Dr. Ohori), and (VI) notable differences in the malpractice climate (please refer to a review by Dr. Poller in this focused series).
North American medical practice, is of a high standard, but it has to contend with higher rates of medical malpractice claims with instances of defensive medical practice due to its severe malpractice climate (26,27,47). The over-diagnosis and overtreatment of equivocal cases may cause significant increases in medical costs as well as harm to the patient (48). This situation is unwelcome elsewhere in the world (49). Clinical guidelines used in North American practice take account of the local malpractice climate and fear of litigation, but perhaps there could be a way of highlighting those aspects of North American professional practice guidelines that are designed primarily for defensive medical purposes, in order to clarify aspects that are primarily or in part designed to avoid potential malpractice litigation, and which may not be necessary in localities where medical malpractice claims are negligible (please refer to a review by Dr. Poller).
The European Association of Nuclear Medicine did not immediately endorse the 2015 ATA management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer (50) and held an interactive international symposium to reach a consensus (51). It is therefore essential to standardize diagnoses, clinical management, treatments, and patient care based on scientific evidence and the best practice for the patient’s sake. The global community of clinicians treating and managing patients should be willing to accept outside points of view, and engage in discussion and debate about areas of controversy and diagnostic or therapeutic disagreement (please refer to reviews by Dr. Kumarasinghe, Dr. Pusztaszeri, Dr. Ito, Dr. Okamoto and Dr. Michael).
Why now?
How should we standardize our clinical approach in the era of the recent breakthrough events in thyroid FNA cytology and thyroid tumor classification? These include the introduction of borderline thyroid tumor entities in the 4th edition WHO classification of tumors of endocrine organs (2) and the second edition of the Bethesda system for reporting thyroid FNA cytology (1), which changed the cytological diagnostic criteria for PTC and incorporated NIFTP, a borderline tumor entity (please refer to a review by Dr. Canberk). Furthermore, over-diagnosis and overtreatment of thyroid carcinoma, in particular papillary microcarcinoma and encapsulated follicular variant of PTC became a worldwide phenomenon in the 2010’s, and a solution became an urgent issue (22,23,52-54). Recent Western clinical guidelines have changed from recommending aggressive cancer treatment (total thyroidectomy followed by RAI ablation) to a more conservative approach for low-risk thyroid carcinomas (3). Thus, it is now time to reconsider standardization of Western and Eastern practice because some of the conservative management methods (so-called active surveillance for low-risk thyroid carcinomas and infrequent use of RAI to treatment thyroid carcinomas) are mainly confined to Asian practice (8,9,46,55) (please refer to reviews by Dr. Ito, Dr. Pusztaszeri, Dr. Nguyen and Dr. Zhu for active surveillance, and by Dr. Okamoto for RAI treatment in this focused series).
How should we adapt to the new era?
As stated above, there were several recent breakthrough events in thyroid FNA cytology and thyroid tumor classification. The historic period in thyroid pathology when pathologists had only two diagnostic choices (benign and malignant) for the diagnosis of thyroid tumors will soon end, and we will enter a new era with three options (benign, borderline, and malignant) similar to other organ systems. In the modern era, pathologists will have to categorize tumors previously termed carcinomas into (I) higher-risk malignancy categories (true carcinoma), which require total thyroidectomy, (II) lower-risk carcinomas for which lobectomy alone is curative, and (III) borderline/precursor tumors for which immediate surgery is not necessary or can be left untreated (8,9,22,23,56). This is in part because pathologists, clinicians, and patients have different concepts of cancer/carcinoma. The pathologist knows that thyroid cancers are heterogeneous ranging from borderline/precursor lesions (simple excision is curative, similar to benign tumors) to lethal malignancies (a wide spectrum of risk of prognosis for recurrence-free or overall survival depending on the cancer subtype and cancer stage), but the majority of clinicians and patients assume that if a label of ‘cancer’ is given it will inevitably progress and metastasize, leading to death (22,23,37,39). In the latter scenario, significant numbers of clinicians recommend cancer therapy (total thyroidectomy followed by RAI ablation) to the patient, and many patients accept it. In shared decision making nearly 80% of patients with small (<2 cm) localized PTCs were historically treated by total thyroidectomy in the United States (54). However, the cancer-specific mortality of these patients was nearly 0%, and these tumors were almost always not biologically aggressive (3,41-45). This was the primary reason why diagnostic terminology has now altered (2,21,25,28,36,39,57). When we trained as pathologists, textbooks stated that the (I) presence of PTC-type nuclear features was diagnostic for PTC and that (II) identification of capsular invasion was diagnostic for malignancy. Therefore, patients with encapsulated non-invasive PTCs or capsular invasion alone FTCs were often treated by total thyroidectomy in the past (58,59). This is incorrect. It is necessary to change to a risk-based classification of thyroid tumors, which is essential for pathologists to reduce overdiagnosis and overtreatment of thyroid carcinomas; however, this may take some time.
Borderline/dysplasia category
Although this is a personal view, Dr. Kakudo has previously proposed a name change from “atypia of undetermined significance” (AUS, TBS Category III) and “follicular neoplasm” (FN, TBS Category IV) to “borderline/dysplasia” to avoid any misunderstanding by clinicians and patients as more than 99% of carcinomas in these categories are low-risk and cured when clinical cancers are excluded (Figure 2) (8,9,56,60,61). It was also intended to justify Asian clinical approach (active surveillance) to AUS and FN thyroid nodules with clinically benign findings. Furthermore, Dr. Kakudo suggests that the borderline/dysplasia cytological categories should no longer be termed “indeterminate”, “uncertain”, or “gray zone” because these classifiers may convey a confusing message to clinicians and patients (7,56,60,61).
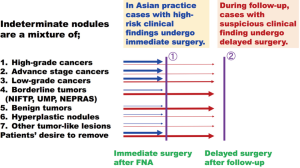
Western pathologists however are unlikely to accept a proposed name change of the TBS Category III/AUS and Category IV/FN categories to a “borderline” category. The cytological appearances of benign, borderline, low-grade, and high-grade thyroid cancer lesions overlaps significantly, confirmed by poor inter-rater kappa scores for diagnosis of TBS Category III/AUS (Thy 3a) and Category IV/FN (Thy 3f) categories (62,63). Rare lethal carcinomas are inevitably included in some TBS Category III/AUS and Category IV/FN cases in Western practice. The proposed name changes from “indeterminate” to “borderline” might cause confusion among Western clinicians and patients as well as an implied risk of malpractice litigation. On the other hand, as the vast majority of thyroid nodules in TBS Category IV/FN category are not lethal cancers in Asian practice, Asian pathologists are aware that risk stratification with other clinical tests and exclusion of clinical cancers by immediate surgery is successful (Figure 2) (7-9,56). This is the reason why patients with TBS Category IV/FN nodules often undergo active surveillance in Asian practice when there are no suspicious clinical findings (Table 2), even if there is a risk cancer cannot be excluded by FNA cytology alone (Figure 2). On the other hand, diagnostic surgery is still favored in significant numbers of centers in Western practice, as it is believed to be the only reliable method to prevent missing malignancy (1,3,58,67,68). Although it may no longer be true in some Western countries. For example, patients with indeterminate thyroid nodules (Bethesda III or IV) are offered a choice between follow-up, diagnostic surgery, repeat FNA with or without molecular testing (shared decision making and personalized management) in some Western centers. With this strategy, a significant proportion of these patients will not have surgery and the risks of malignancy (ROMs) at resection will be significantly higher than the ones of the Bethesda system, probably approaching the ones in Asian countries (see a review by Pusztaszeri et al. in this focused series).

Full table
Clinical management of TBS Category IV/FN nodules recommended by the Japan Thyroid Association
From the cytological report of TBS Category IV/FN, surgeons and endocrinologists in Japan apply other clinical features useful for decision-making in clinical management of patients (7-9,56,64), similar to Asian practice (please see reviews by Dr. Ito, Dr. Nguyen and Dr. Zhu in this focused series). This is because FNA cytology alone cannot efficiently select patients with thyroid nodules for surgery because the ROM of TBS Category IV/FN nodules is within the range of baseline ROM, which was estimated to be 10–20% in Western countries (58,67). Japanese surgeons and endocrinologists regard the ROM in patients with TBS Category IV/FN nodules as not being sufficiently high to offer surgery to all patients without further selection, whereas surgery was always recommended for patients with TBS Category IV/FN by most guidelines in Western countries in the past (1,3,58,59,67,68).
As strict triage of TBS Category IV/FN nodules for surgery (large nodule >4 cm, compression symptoms, suspicious ultrasound features, positive lymph node metastasis, high serum thyroglobulin, patient desire, etc.) is carried out in Asian practice (Table 2), the resection rates of TBS Category IV/FN nodules are usually lower and ROM of TBS Category IV/FN nodules is usually higher than those in Western practice (please also see reviews by Asian WG members Dr. Abelardo, Dr. Chen, Dr. Ooi, Dr. Zhu, Dr. Liu, Dr. Tangnuntachai and Dr. Ngnuyen in this focused series) (8,9,17,18). Therefore, cases of TBS Category IV/FN nodules under clinical follow-up mostly include low-risk carcinomas (T1 or T2, ex0, N0, and M0), borderline tumors, and benign lesions, with very rarely cases of lethal cancer or advanced stage carcinoma when patients do not accept surgery (Figure 2) (8,9).
Clinical management of thyroid nodules recommended by the 2015 ATA clinical guidelines
To reduce overdiagnosis and overtreatment of thyroid carcinomas, the 2015 ATA clinical guidelines introduced strategies using multidisciplinary approaches, including molecular tests (3) (please refer to reviews by Dr. Pusztaszeri and Dr. Ohori in this focused series). They are: (I) do not perform thyroid FNA on nodules smaller than 1 cm, (II) clinical follow-up instead of diagnostic surgery for TBS Category III/AUS and TBS Category/IV FN nodules when molecular tests reveal no suspicious features, and (III) restrict surgery to lobectomy and avoid RAI for low-risk thyroid carcinomas. These strategies are intended to reduce overdiagnosis and overtreatment for benign nodules, borderline/precursor tumors, and low-risk carcinomas, although some authors question the diagnostic performance of the gene expression classifier (69) (please refer to reviews by Dr. Zhu and Dr. Ohori). Most importantly these molecular tests are commercially-based and expensive, and are not generally available outside of North America (please refer to reviews by Dr. Chen, Dr. Choden, Dr. Ooi, Dr. Liu and Dr. Abelardo). Whether this strategy recommended by the ATA is equally valid to reduce over-diagnosis and overtreatment of thyroid tumors will soon be confirmed, as the rate of total thyroidectomy for small (<2 cm) localized thyroid carcinomas was high (>80%) in 2014 in the USA (53) and approximately 30% of patients with low-risk thyroid carcinoma still received RAI in 2015 in the USA (53,70), which was in many cases unnecessary (3,59,71-73) (please refer to reviews by Dr. Okamoto and Dr. Ito).
Different practice patterns and different ways of thinking
NIFTP practice
NIFTP is considered to require surgery in Western practice because it is a precursor tumor and accurate preoperative diagnosis has not been established (1,2,28,68,73), and a few cases with metastasis have been reported (74,75). Under Western logic and clinical practice, the estimated ROMs in patients with TBS Category IV/FN nodules are too high for observation and so the majority (often more than 70%) of patients accept surgery for diagnostic purposes (1,3,8,9,17,18,54,63,67,73). As NIFTPs and TBS Category IV/FN nodules are treated by surgery in Western practice, a significant proportion (>30%) of TBS Category IV/FN nodules are NIFTP on histology (1,2,76-79) (please refer to reviews by Dr. Kumarasinghe and Dr. Canberk). On the other hand, in Japan and elsewhere in Asia, close follow-up or active surveillance is acceptable as a management option for patients with TBS Category IV/FN nodules (8,9,44,49,62), thus borderline tumors are rare on histology in Asia (9,10,13,14,17,18). Japanese clinicians believe that surveillance rather than diagnostic lobectomy better identifies potential lethal malignancies, and clinically significant malignancies are rarely missed employing this conservative practice (Figure 2) (7,9,56,64,65) (please refer to a review by Dr. Ito). As active surveillance for patients with TBS Category IV FN nodules significantly reduces rates of surgical treatment for borderline tumors, low resection rates and high ROMs for TBS Category IV FN nodules are achieved in Asian practice (9,17,18,64,65) (please refer to reviews by Dr. Liu, Dr. Nguyen, Dr. Chen, Dr. Zhu, Dr. Ooi, Dr. Tangnuntachai, Dr. Abelardo and Dr. Guleria). Unnecessary treatment-related complications in patients who may not require treatment are therefore reduced, which is an indispensable factor for Asian practice (7-9,64,65). Please read a review by Pusztaszeri et al. in this focused series describing when active surveillance was introduced to a Western (Canadian) patient cohort.
Active surveillance for low-risk papillary microcarcinomas
Observation without surgical treatment is practiced for patients with low-risk papillary microcarcinoma in several medical centers in Japan and Korea (80-85). Based on these studies, less than 15% of low-risk papillary microcarcinomas grow by 3 mm or more in diameter or develop lymph node metastasis during follow-up, and most importantly, no thyroid cancer death was reported among more than 1,000 patients for more than 10 years of follow-up (80-83). The active surveillance instead of immediate surgery were currently selected for more than 50% of patients with microcarcinomas in Japan (83) (please refer to reviews by Dr. Ito in this focused series). Expert thyroid pathologists proposed referring to it as papillary microtumor instead of carcinoma (57), and a proposal to classify papillary microcarcinoma in the borderline tumor category was reported by Kakudo et al. (37,39,44,45). Recently, Western practice also moved towards a more conservative approach for small low-risk PTCs and active surveillance (non-surgical approach) became a management choice for small low-risk PTCs (86,87) approved by the ATA clinical guidelines (3).
ROM in Asian practice
As to ROMs of TBS cytological categories, Vuong et al. showed in their excellent meta-analysis, that ROM of resected nodules was significantly higher in most TBS categories in Asian practice compared to those in Western practice, except the malignant category which was equal between two geographic areas (18). The underlying factors which create the differences appear to be (I) different prevalence rates of cancer, (II) variable indications for FNA for thyroid nodules and (III) different practice patterns for low-risk thyroid carcinoma and borderline thyroid tumors between the two geographic regions (8,9,66) (please examine reviews by Dr. Gueleria, Dr. Nguyen and Dr. Ooi in this focussed issue).
In a previous study, a trend for higher ROMs for TBS categories was found in practices with higher ROMs for all resected nodules (9). Although exact disease prevalence at each hospital is not known, we can assume the prevalence based on the ROMs for all resected nodules. In the meta-analysis by Vuong et al., the ROMs for all resected nodules were higher in Asian practice than those of Western practice. Thirteen percent of all aspirated nodules in Asian countries were found to be malignant while only 8.1% were malignant in Western countries (18), which may explain one factor for the higher ROMs in Asian practice. The second factor may be the selection of nodules undergoing FNA, also an important pre-test factor. The overall ROM will be increased for the TBS categories if only more concerning thyroid nodules are aspirated in Asian countries in general as compared to Western countries (please see this pre-test selection in reviews by Dr. Zhu and by Dr. Liu in this focused series). As an example of pre-test bias, the ATA clinical guidelines for cystic thyroid nodules recommend not to aspirate pure cystic nodules less than 2 cm in size. This was originally designed to reduce unnecessary repeat FNAs on TBS Category I inadequate cytological diagnoses on cyst fluid only samples, but this pre-test selection of the cystic thyroid nodules results in higher ROM for cyst fluid only samples in the Western practice (please refer to a review by Dr. Hirokawa). The third factor is likely to be the different practice patterns for low risk thyroid carcinoma and borderline thyroid tumors between the two geographic regions, as explained and discussed in the section I above.
Entire sampling of encapsulated thyroid nodules
We are entering a new era with three diagnostic options (benign, borderline, and malignant) in thyroid tumor classification, similar to other organ systems, instead of two choices (benign and malignant); however, many pathologists still believe the old concept that benign (FA) and malignant (carcinoma) thyroid tumors by can be identified based on invasion. This was based on an incorrect belief that all malignant FTCs have detectable invasion on histopathological examination. This concept is borne from rare exceptional cases that develop metastasis (88) without detectable invasion even after sampling of the entire tumor capsule (89) or the absence of a primary tumor in the thyroid gland (37,90). However, there are two different approaches to this: Western pathologists recommend sampling the entire tumor interface to identify any evidence of malignancy and to defend their practice from malpractice litigation (2,28,91), whereas Asian pathologists take more samples only in equivocal cases and consider sampling of the entire thyroid nodule to be unnecessary (44,92).
Conclusions
The introduction of borderline tumors to thyroid tumor classification is an epoch-making change in thyroid pathology practice. Thyroid tumors are classified into three risk groups by the probability of recurrence/metastasis, negligible risk (<0.1%) in benign tumors, very low-risk (<1%) in borderline tumors, and high-risk in malignant tumors. As the ATA clinical guidelines divide thyroid carcinomas into 3 risk groups (low-, intermediate-, and high-risk) in 2016 for structural disease recurrence (3), pathologists can risk-stratify thyroid tumors into five risk groups, (I) benign tumors (<0.1% risk), (II) borderline tumors (<1% risk), (III) low-risk carcinomas (1–10%), (IV) intermediate-risk carcinomas (10–20%), and (V) high-risk carcinomas (20–50%) morphologically. Furthermore, the Japan Association of Endocrine Surgeons/Japanese Society of Thyroid Surgery guidelines classified PTC into three risk groups (very low or low-risk, intermediate-risk, and high-risk) by the probability for cancer death in 2014 and was revised in 2018 (46,55,64), pathologists can risk-stratify PTCs into five risk groups for cause-specific survival, (I) benign tumors (100%), (II) borderline tumors (100%), (III) very-low and low-risk carcinomas (100% at 15 years), (IV) intermediate-risk carcinomas (99% at 15 years), and (V) high-risk carcinomas (92% at 15 years) (45,46). The editor favors this risk stratification of thyroid tumors (37-39,44,45). This risk stratification also suggests that all thyroid tumors have some potential for recurrence/metastasis. The editor would like to emphasize that this new concept protects pathologists from failing to diagnose malignancy and malpractice litigation. The editor hopes these proposed changes to thyroid tumor classification and the reporting system for thyroid FNA cytopathology in this editorial will reduce the fears of litigation and reduce unnecessary defensive practice by pathologists. The editor has a dream that the East and West will meet together, and have more standardized clinical approaches to thyroid nodules based on real scientific evidence as to the best practice for the patient (7-9,45,49,55,56,60,61).
Acknowledgments
The editor thanks all members of the Asian Working Group in Thyroid Cytology/Pathology and all authors of this focused series for their valuable contributions, which made it possible to clarify differences between Asian and Western thyroid practice.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gland Surgery for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences”. This article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-2019-catp-02). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. KK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gland Surgery from Sep 2018 to Aug 2020.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ali S, Cibas E. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. 7nd ed. New York: Springer, 2017.
- Lloyd RV, Osamura RY, Klöppel G, et al. editors. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: IARC, 2017.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Kakudo K, Katoh R, Sakamoto A, et al. Thyroid gland: international case conference. Endocr Pathol 2002;13:131-4. [Crossref] [PubMed]
- Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 2002;26:1508-14. [Crossref] [PubMed]
- Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 2004;28:1336-40. [Crossref] [PubMed]
- Kakudo K. Supplemental Chapter 2: How to follow fine-needle aspiration biopsy-proven benign thyroid nodules, active surveillance vs diagnostic lobectomy for indeterminate nodules and individualized treatments for low-risk thyroid carcinomas. In: Kakudo K. editor. Thyroid FNA cytology, differential diagnoses and pitfalls. 1st ed. Himeji-city: BookWay Global, 2016:283-92.
- Kakudo K. How to handle borderline/precursor thyroid tumors in management of patients with thyroid nodules. Gland Surg 2018;7:S8-18. [Crossref] [PubMed]
- Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice – Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. [Crossref] [PubMed]
- Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid 2017;27:983-4. [Crossref] [PubMed]
- Kakudo K, Liu Z, Bychkov A, et al. Chapter 21: Nuclear features of papillary thyroid carcinoma (BRAF-like tumors), non-invasive follicular thyroid neoplasm with papillary-like nuclear features (RAS-like tumors) and follicular adenoma/follicular thyroid carcinoma (RAS-like tumors). In: Kakudo K. editor. Thyroid FNA cytology, differential diagnoses and pitfalls. 2nd ed. Singapore: Springer, 2019:173-9.
- Bychkov A, Kakudo K, Hong SW. Current thyroid FNA practices in Asia – A missing voice. J Pathol Transl Med 2017;51:517-20. [Crossref] [PubMed]
- Bychkov A, Keelawat S, Agrawal S, et al. Impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology 2018;50:411-7. [Crossref] [PubMed]
- Bychkov A, Jung CK, Liu Z, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol 2018;29:276-88. [Crossref] [PubMed]
- Agarwal S, Bychkov A, Jung CK, et al. The prevalence and surgical outcomes of Hürthle cell lesions in fine‐needle aspirates of the thyroid: a multi-institutional study in six Asian countries. Cancer Cytopathol 2019;127:181-91. [Crossref] [PubMed]
- Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary like nuclear features in Asian practice. Pathol Int 2019;69:202-10. [Crossref] [PubMed]
- Vuong HG, Thao TK, Bychkov A, et al. Clinical impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the risk of malignancy in the Bethesda system for reporting thyroid cytopathology: a meta-analysis of 14,153 resected thyroid nodules. Endocr Pract 2019;25:491-502. [Crossref] [PubMed]
- Vuong HG, Ngo HTT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol 2020;128:238-49. [Crossref] [PubMed]
- Lai WA, Hang JF, Liu CY, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch 2020;476:431-7. [Crossref] [PubMed]
- Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms-an observer variation study. Endocr Pathol 2020;31:132-40. [Crossref] [PubMed]
- Ohba K, Mitsutake N, Matsuse M, et al. Encapsulated papillary thyroid tumor with delicate nuclear changes and a KRAS mutation as a possible novel subtype of borderline tumor. J Pathol Transl Med 2019;53:136-41. [Crossref] [PubMed]
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605-13. [Crossref] [PubMed]
- Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 2014;15:e234-42. [Crossref] [PubMed]
- Rosario PW. Noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS) or encapsulated papillary thyroid carcinoma (PTC). J Pathol Transl Med 2020;54:263-4. [Crossref] [PubMed]
- Jung CK, Lee Y, Park SY, et al. New insights into classification and risk stratification of encapsulated thyroid tumor with a predominantly papillary architecture. J Pathol Transl Med 2020;54:197-203. [Crossref] [PubMed]
- Renshaw AA, Gould EW. Why there is the tendency to "overdiagnose" the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2002;117:19-21. [Crossref] [PubMed]
- Ohori NP. FNA cytopathology and molecular test characteristics in the changing landscape of papillary thyroid carcinoma. J Basic Clin Med 2015;4:103-9.
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma. A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2:1023-9. [Crossref] [PubMed]
- Nabhan F, Porter K, Lupo MA, et al. Heterogeneity in positive predictive value of RAS mutations in cytologically indeterminate thyroid nodules. Thyroid 2018;28:729-38. [Crossref] [PubMed]
- Lee SE, Hwang TS, Choi YL, et al. Molecular profiling of papillary thyroid carcinoma in Korea with a high prevalence of BRAFV600E mutation. Thyroid 2017;27:802-10. [Crossref] [PubMed]
- Liang J, Cai W, Feng D, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol 2018;244:215-26. [Crossref] [PubMed]
- Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90:6373-9. [Crossref] [PubMed]
- Liu RT, Chen YJ, Chou FF, et al. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf) 2005;63:461-6. [Crossref] [PubMed]
- Kim TY, Kim WB, Song JY, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf) 2005;63:588-93. [Crossref] [PubMed]
- Ito Y, Yoshida H, Maruo R, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J 2009;56:89-97. [Crossref] [PubMed]
- Williams ED. Guest Editorial: Two Proposals Regarding the Terminology of Thyroid Tumors. Int J Surg Pathol 2000;8:181-3. [Crossref] [PubMed]
- Kakudo K, Bai Y, Liu Z, et al. Classification of thyroid follicular cell tumors: with special reference to borderline lesions. Endocr J 2012;59:1-12. [Crossref] [PubMed]
- Liu Z, Zhou G, Nakamura M, et al. Encapsulated follicular thyroid tumor with equivocal nuclear changes, so called well-differentiated tumor of uncertain malignant potential: a morphological, immunohistochemical and molecular appraisal. Cancer Sci 2011;102:288-94. [Crossref] [PubMed]
- Kakudo K, Bai Y, Liu Z, et al. Encapsulated papillary thyroid carcinoma, follicular variant: a misnomer. Pathol Int 2012;62:155-60. [Crossref] [PubMed]
- Nickel B, Howard K, Brito JP, et al. Association of preferences for papillary thyroid cancer treatment with disease terminology. A discrete choice experiment. JAMA Otolaryngol Head Neck Surg 2018;144:887-96. [Crossref] [PubMed]
- Bai Y, Kakudo K, Li Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci 2008;99:1908-15. [PubMed]
- Piana S, Frasoldati A, Di Felice E, et al. Encapsulated well-differentiated follicular-patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol 2010;34:868-72. [Crossref] [PubMed]
- Goffredo P, Cheung K, Roman SA, et al. Can minimally invasive follicular thyroid cancer be approached as a benign lesion? Ann Surg Oncol 2013;20:767-72. [Crossref] [PubMed]
- Kakudo K, El-Naggar AK, Hodak SP, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int 2018;68:327-33. [Crossref] [PubMed]
- Kakudo K, Wakasa T, Ohta Y, et al. Prognostic classification of thyroid follicular cell tumors using Ki-67 labeling Index: How to report high-risk thyroid carcinomas? Endocr J 2015;62:1-12. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Oda H, et al. Appropriateness of the revised Japanese guidelines' risk classification for the prognosis of papillary thyroid carcinoma: a retrospective analysis of 5,845 papillary thyroid carcinoma patients. Endocr J 2019;66:127-34. [Crossref] [PubMed]
- Warrick J, Lengerich E. Letter to the Editor. Thyroid cancer overdiagnosis and malpractice climate. Arch Pathol Lab Med 2019;143:414-5. [Crossref] [PubMed]
- Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PLoS One 2017;12:e0181970. [Crossref] [PubMed]
- Kakudo K, Bychkov A. Malpractice climate is a key difference in thyroid pathology practice between North America and the rest of the world. Arch Pathol Lab Med 2019;143:1171. [Crossref] [PubMed]
- Verburg FA, Aktolun C, Chiti A, et al. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 2016;43:1001-5. [Crossref] [PubMed]
- Luster M, Aktolun C, Amendoeira I, et al. European perspective on 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: proceedings of an interactive international symposium. Thyroid 2019;29:7-26. [Crossref] [PubMed]
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. 2014 Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Welch HG, Doherty GM. Saving thyroids - overtreatment of small papillary cancers. N Engl J Med 2018;379:310-2. [Crossref] [PubMed]
- Takami H, Ito Y, Okamoto T, et al. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg 2014;38:2002-10. [Crossref] [PubMed]
- Kakudo K, Bychkov A. Letter to Editor. Cytologically borderline thyroid nodules as a key target to reduce overdiagnosis and overtreatment of thyroid cancer. Arch Pathol Lab Med 2019;143:784-5. [Crossref] [PubMed]
- Rosai J. Renaming papillary microcarcinoma of the thyroid gland: the Porto proposal. Int J Surg Pathol 2003;11:249-51. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Freeman JL. Surgical management of thyroid nodular disease-personal evolution throughout 4 decades of practice. JAMA Otolaryngol Head Neck Surg 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Kakudo K. Chapter 1: Factors impacting thyroid fine needle aspiration cytology and the algorithm for cytological diagnosis. In: Kakudo K. editor. Thyroid FNA cytology, differential diagnoses and pitfalls. 1st ed. Himeji-city: BookWay Global, 2016:15-22.
- Kakudo K, Pusztaszeri M, Bongiovanni M. Chapter 2: Factors impacting thyroid fine needle aspiration cytology and algorithm for cytological diagnosis in the Japanese system for reporting FNA cytology. In: Kakudo K. editor. Thyroid FNA cytology, differential diagnoses and pitfalls. 2nd ed. Singapore: Springer, 2019:19-26.
- Kocjan G, Chandra A, Cross PA, et al. The interobserver reproducibility of thyroid fine-needle aspiration using the UK Royal College of Pathologists' classification system. Am J Clin Pathol 2011;135:852-9. [Crossref] [PubMed]
- Cibas ES, Baloch ZW, Fellegara G, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med 2013;159:325-32. [Crossref] [PubMed]
- The Japan Thyroid Association, Guidelines for Clinical Practice for the management of Thyroid Nodules in Japan 2013. Tokyo: Nankodo Co., Ltd., 2013:1-277.
- Kihara M, Hirokawa M, Ito Y, et al. Final pathology findings after immediate or delayed surgery in patients with cytologically benign or follicular thyroid nodules. World J Surg 2011;35:558-62. [Crossref] [PubMed]
- Kakudo K, Kameyama K, Miyauchi A, et al. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J 2014;61:539-52. [Crossref] [PubMed]
- Alexander EK. Approach to the patient with a cytologically indeterminate thyroid nodule. J Clin Endocrinol Metab 2008;93:4175-82. [Crossref] [PubMed]
- Ferris RL, Nikiforov Y, Terris D, et al. AHNS Series: Do you know your guidelines? AHNS endocrine section consensus statement: State-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Head Neck 2018;40:1881-8. [Crossref] [PubMed]
- Valderrabano P, Hallanger-Johnson JE, Thapa R, et al. Comparison of postmarketing findings vs the initial clinical validation findings of a thyroid nodule gene expression classifier: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2019. [Crossref] [PubMed]
- Park KW, Wu JX, Du L, et al. Decreasing use of radioactive iodine for low-risk thyroid cancer in California, 1999 to 2015. J Clin Endocrinol Metab 2018;103:1095-101. [Crossref] [PubMed]
- Hodak S, Tuttle RM, Maytal G, et al. Changing the cancer diagnosis: The case of follicular variant of papillary thyroid cancer-Primum non nocere and NIFTP. Thyroid 2016;26:869-71. [Crossref] [PubMed]
- Schnadig VJ. Overdiagnosis of thyroid cancer: Is this not an ethical issue for pathologists as well as radiologists and clinicians? Arch Pathol Lab Med 2018;142:1018-20. [Crossref] [PubMed]
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2017;27:481-3. [Crossref] [PubMed]
- Cho U, Mete O, Kim MH, et al. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 2017;30:810-25. [Crossref] [PubMed]
- Eskander A, Hall SF, Manduch M, et al. A population-based study on NIFTP incidence and survival: Is NIFTP really a "benign" disease? Ann Surg Oncol 2019;26:1376-84. [Crossref] [PubMed]
- Maletta F, Massa F, Torregrossa L, et al. Cytological features of "noninvasive follicular thyroid neoplasm with papillary-like nuclear features" and their correlation with tumor histology. Hum Pathol 2016;54:134-42. [Crossref] [PubMed]
- Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol 2016;146:373-7. [Crossref] [PubMed]
- Strickland KC, Vivero M, Jo VY, et al. Preoperative cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a prospective analysis. Thyroid 2016;26:1466-71. [Crossref] [PubMed]
- Yang GCH, Fried KO, Scongnamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol 2017;45:533-41. [Crossref] [PubMed]
- Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 2010;34:1222-31. [Crossref] [PubMed]
- Miyauchi A, Kudo T, Ito Y, et al. Natural history of papillary thyroid microcarcinoma: Kinetic analyses on tumor volume during active surveillance and before presentation. Surgery 2019;165:25-30. [Crossref] [PubMed]
- Sugitani I, Ito Y, Miyauchi A, et al. Active surveillance versus immediate surgery: Questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid 2019;29:1563-71. [Crossref] [PubMed]
- Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center’s experience in Korea. J Clin Endocrinol Metab 2017;102:1917-25. [Crossref] [PubMed]
- Jeon MJ, Kim WG, Kwon H, et al. Clinical outcomes after delayed thyroid surgery in patients with papillary thyroid microcarcinoma. Eur J Endocrinol 2017;177:25-31. [Crossref] [PubMed]
- Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143:1015-20. [Crossref] [PubMed]
- Lowenstein LM, Basourakos SP, Williams MD, et al. Active surveillance for prostate and thyroid cancers: evolution in clinical paradigms and lessons learned. Nat Rev Clin Oncol 2019;16:168-84. [Crossref] [PubMed]
- Ito Y, Yabuta T, Hirokawa M, et al. Distant and lymph node metastases of thyroid nodules with no pathological evidence of malignancy: a limitation of pathological examination. Endocr J 2008;55:889-94. [Crossref] [PubMed]
- Glomski K, Nose V, Faquin WC, et al. Metastatic follicular thyroid carcinoma and the primary thyroid gross examination: Institutional review of cases from 1990 to 2015. Endocr Pathol 2017;28:177-85. [Crossref] [PubMed]
- Xu B, Scognamiglio T, Cohen PR, et al. Metastatic thyroid carcinoma without identifiable primary tumor within the thyroid gland: a retrospective study of a rare phenomenon. Hum Pathol 2017;65:133-9. [Crossref] [PubMed]
- Ghossein R, Barletta J, Bullock M, et al. Carcinoma of the Thyroid Histopathology Reporting Guide. Sydney: International Collaboration on Cancer Reporting, 2019.
- Kakudo K, Li Y, Taniguchi E, et al. Follicular neoplasms in the 4th edition WHO classification of endocrine organs. J Basic Clin Med 2017;7:10-9.



