The evolving breast reconstruction: from latissimus dorsi musculocutaneous flap to a propeller thoracodorsal fasciocutaneous flap
Background
The publication of the Latissimus dorsi musculocutaneous flap without muscle by Angrigiani et al. in 1995 (1) was in fact the first thoracodorsal artery perforator flap (TAP/TDAP-flap) and represented a new way of thinking in autologous flap design based on the angiosome concept previously presented by Taylor and Palmer in 1987 (2). The TAP or TDAP-flap concept within breast surgery has since been developed further by Hamdi et al. for both oncoplastic breast surgery as well as for breast reconstruction (3,4) and a muscle sparing variation of the technique in combination with an implant was subsequently published by Brackley et al. (5).
In 2013 the concept was taken a step further, when the propeller TAP-flap was combined with the hammock technique using an ADM and an implant for a one stage breast reconstruction (6). In this issue of GS, Angrigiani et al. show that the propeller TAP-flap can be designed in an oblique upwards design, enabling a flap length of more than 30 cm, and furthermore that the dominating perforator, in some instances runs anterior to the latissimus dorsi muscle straight to the subcutis (7). The applicability of the propeller TAP-flap in reconstructive breast surgery is thus expanding and includes an array of indications from corrective oncoplastic breast procedures to one stage breast reconstruction. The aim of this editorial is to give an update on the use of the propeller TAP-flap within the field of breast reconstruction. To emphasize its simplicity and applicability focus will be on the planning and surgical technique, as well as the debate on LD vs. TAP and future perspectives.
Preoperative planning and surgical technique
TAP perforators are quite predictably localized in up to 80% of patients approximately 8 cm from the top of the axilla and close to the anterior edge of the LD muscle (1,3-7). In general perforators can reliably be localized based on anatomical knowledge and intraoperative exploration (8). This also applies for the thoracodorsal perforator, however, pre-operative identification is recommended by most authors using either a Doppler probe, color Doppler ultrasonography or CT-angiography (6,9,10). The Doppler probe enables identification of the location of the perforators, but does not give any additional information other than an estimation of the relative size of the perforators based on the volume of sound. In contrast Color Doppler ultrasonography and CT-angiography not only visualize the location of the perforators, but also provides an estimate of the vessel diameter for each perforator as well as information about topography and direction of their branches (6,10). This information increases the surgeons comfort level and speeds up the dissection (10,11). The advantage of color Doppler ultrasonography in particular is that it can be performed either immediately before or during surgery. The disadvantage is that it requires some experience to perform. CT-angiography has been shown to reduce procedure time (10,11). However it is time consuming, costly and requires dedicated radiologists. The simplest and most optimal setup seems to be a well trained surgeon experienced with use of either a Doppler probe or color Doppler ultrasonography to identify the perforators before surgery (6).
The location of the perforators is variable and it seems that in 1/15 patients the dominant perforator originates from the horizontal branch of the thoracodorsal vessel. When this is the case, the perforator is approximately 4-5 cm behind the anterior edge of the LD muscle and dissection of the perforator is necessary and in some instances the horizontal branch needs to be divided to gain sufficient length (6). Another variation that calls for an alternative muscle-sparing design is the case with several small perforators instead of 1-3 larger vessels.
The TAP-flap can be dissected by a combined use of a monopolar cautery and a scalpel. Microsurgical instruments are generally not needed. Loop-magnification can be an advantage for perforator dissection but is usually not required when the perforator location is mapped prior to surgery. Bipolar cautery can be used to peel off the muscle fibers from around the perforator if necessary.
Flap design variations
The propeller TAP-flap skin paddle can be designed in many different ways, but three designs have been published: (I) an oblique upwards design in a cranial direction ending medially to the scapula (7); (II) a horizontal design (3,4); (III) an oblique downward design in a caudal direction, where the skin paddle is designed within the boundaries of the LD muscle following the upper edge of the muscle (6) (Figure 1).
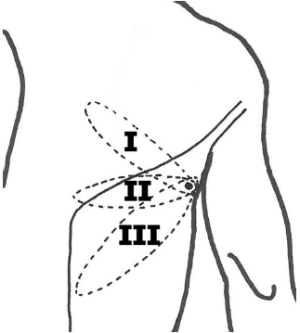
All of these designs leave a satisfying donor site scarring, which can be hidden by clothing. However, there seems to be a significant difference in terms of the complexity of perforator dissection required according to the description of the three different designs. Design I and II require a longer axis of rotation of 180 degrees or more, whereas design III generally makes the angle less than 135 degrees. Thus, designs I and II requires dissection of the perforator to the TD-vessels to gain sufficient laxity when rotating the perforator, whereas identification of the perforator at the fascia level is usually sufficient to allow the required rotation when the oblique downwards design III is used. The disadvantage of the limited dissection in this design is, however an occational lateral bulkiness in the axilla, easily corrected by liposuction along with a secondary procedure. The dissection of the oblique downward design III is quick, easy, and simple and vascular compromise is seldom a problem. However, dissection of the perforator can be argued to be advantageous for better shaping of the reconstruction.
LD versus TAP-flap
The Latissimus dorsi-flap (LD-flap) is a good and reliable option for breast reconstruction, either combined with an implant or alone as an extended flap (12-14). LD-reconstructions have been criticized for the morbidity of the muscle loss and alleged high rate of complications. Most commonly reported are seroma formation at the donor site, postoperative shoulder dysfunction, and/or pain in the affected upper extremity (14-16). The long term morbidity has been and will continue to be debated in literature (17). Surgeons seem to adapt to two different view-points, one stating that there are no problems in regard to donor site morbidity as long as the patient, guided by physiotherapist continue to use the remaining muscles of the shoulder cuff. The other group is reluctant to use the LD-flap as a reconstructive option stating that the morbidity is too high for the use to be justified. It is probably true that patients can achieve a fairly normal function of their muscle and arm with sufficiently guided rehabilitation. However, in our experience, LD reconstructed patients report some impairment of shoulder and arm function when asked at 5 or 10 years follow-up. Furthermore, when inspected, the spine seems to curve towards the non-operated side in many of these patients.
The TAP-flap does not impair the function of the shoulder or arm as opposed to the LD-flap, since the muscle and neural innervations are totally spared (18). The morbidity, both perioperative and long term, seem to be very low regarding back seroma formation and function of the affected upper extremity. In select cases reconstruction can be achieved by the TAP-flap alone or in combination with fat grafting with the flap working as a vascular matrix (19). In most cases an implant is needed to achieve the reconstructive goals (6) (Figure 2). The use of implants is not without morbidity and implant exchange and re-operation related to capsular contracture are to be expected. The experience is limited so far, but rates will probably be similar to those of an LD in combination with an implant (20).
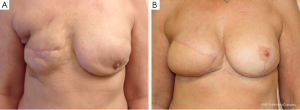
The paper by Angrigiani et al. in this issue of GS is yet another step toward a better understanding of the potentials and limitations of the use of the TAP-flap for breast reconstruction (7).
Future aspects
The TAP-flap seems to be a promising tool for oncoplastic and reconstructive breast surgery and will certainly become an invaluable addition to breast reconstructive methods. Reports of its use by several different surgeons provide us with the diversity of opinions needed for objective evaluation. Experience is still limited and long term results are awaited. How does it affect the patients in terms of long term aesthetic satisfaction, quality of life, shoulder and arm functionality? A constantly changing environment of breast surgery calls for plasticity and diversity. A prospective randomized trial is needed to evaluate the true impact of the TAP-flap in context of other reconstructive methods.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [PubMed]
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. [PubMed]
- Hamdi M, Van Landuyt K, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg 2008;121:1632-41. [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [PubMed]
- Brackley PT, Mishra A, Sigaroudina M, et al. Modified muscle sparing latissimus dorsi with implant for total breast reconstruction - extending the boundaries. J Plast Reconstr Aesthet Surg 2010;63:1495-502. [PubMed]
- Thomsen JB, Bille C, Wamberg P, et al. Propeller TAP flap: is it usable for breast reconstruction? J plast Surg Hand Surg 2013;47:379-82.
- Angrigiani Claudio, Rancati Alberto, Escudero Ezequiel, et al. Propeller thoracodorsal artery perforator flap for breast reconstruction. Gland Surg 2014;3:174-80.
- Gunnarsson GL, Jackson IT, Thomsen JB. Freestyle facial perforator flaps—a safe reconstructive option for moderate-sized facial defects. Eur J Plast Surg 2014;37:315-8. [PubMed]
- Yu P, Youssef A. Efficacy of the handheld Doppler in preoperative identification of the cutaneous perforators in the anterolateral thigh flap. Plast Reconstr Surg 2006;118:928-33. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [PubMed]
- Whitaker IS, Smit JM, Rozen W, et al. Pre operative computed tomographic angiography (CTA): a valuable lesson in planning DIEP flaps. J Plast Reconstr Aesthet Surg 2009;62:551. [PubMed]
- Lindegren A, Halle M, Docherty Skogh AC, et al. Postmastectomy breast reconstruction in the irradiated breast: a comparative study of DIEP and latissimus dorsi flap outcome. Plast Reconstr Surg 2012;130:10-8. [PubMed]
- Dini M, Quercioli F, Mori A, et al. Expanding the indications for latissimus dorsi musculocutaneous flap in totally autologous breast reconstruction: the extended variant. Ann Surg Oncol 2011;18 Suppl 3:S266-70; author reply S271.
- Button J, Scott J, Taghizadeh R, et al. Shoulder function following autologous latissimus dorsi breast reconstruction. A prospective three year observational study comparing quilting and non-quilting donor site techniques. J Plast Reconstr Aesthet Surg 2010;63:1505-12. [PubMed]
- Kim H, Wiraatmadja ES, Lim SY, et al. Comparison of morbidity of donor site following pedicled muscle-sparing latissimus dorsi flap versus extended latissimus dorsi flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:640-6. [PubMed]
- Forthomme B, Heymans O, Jacquemin D, et al. Shoulder function after latissimus dorsi transfer in breast reconstruction. Clin Physiol Funct Imaging 2010;30:406-12. [PubMed]
- Koh CE, Morrison WA. Functional impairment after latissimus dorsi flap. ANZ J Surg 2009;79:42-7. [PubMed]
- Hamdi M, Decorte T, Demuynck M, et al. Shoulder function after harvesting a thoracodorsal artery perforator flap. Plast Reconstr Surg 2008;122:1111-7. [PubMed]
- Santanelli F, Longo B, Germano S, et al. Total breast reconstruction using the thoracodorsal artery perforator flap without implant. Plast Reconstr Surg 2014;133:251-4. [PubMed]
- Hardwicke JT, Prinsloo DJ. An analysis of 277 consecutive latissimus dorsi breast reconstructions: a focus on capsular contracture. Plast Reconstr Surg 2011;128:63-70. [PubMed]


