
The implementation of enhanced recovery after surgery protocols in ovarian malignancy surgery
Introduction
The enhanced recovery after surgery (ERAS), which is also known as fast-track surgery, refers to multimodal interventions to reduce the length of hospital stay and complications through alleviating the surgical stress response that patients experience before, during and after surgical procedures (1). This includes procedures ranging from preoperative counselling, avoidance of bowel preparations, shortening of preoperative fasting, tailored anesthesia and analgesia, avoidance of drains, early postoperative feeding and mobilization. This concept of a multimodal approach was first developed in Denmark by Kehlet and his group (2). These colorectal surgeons described a “stress-free” colonic resection for colorectal cancer patients by adopting minimally-invasive surgical procedures, epidural analgesia, and postoperative early feeding and mobilization. They further developed ERAS by initiating clinical studies and organizing educational symposia, leading up to the formal foundation of the ERAS Society. The principles developed by the ERAS Society were incorporated into elective colonic surgery and have been embraced by other surgical disciplines such as orthopedics, thoracic surgery, urology and gynecology (3-5).
The ERAS Society has published guidelines for perioperative care of gynecological oncology patients in 2016 (6,7), and recently updated a number of components in its newer version in 2019 (8). The ERAS gynecologic oncology guidelines include 20 elements that are recommended to be followed before, during and after gynecologic surgery (Table 1). However, the evidence to support the guidelines has been mainly derived from either observational studies or based on findings from other surgical disciplines. Studies regarding individual ERAS component in gynecological oncology are limited by the heterogeneity in the design of individual studies, composition of specific interventions, study participants and wide range of surgical procedures from minimally-invasive surgery to cytoreductive surgery with multiple organ resections for advanced ovarian cancer (9).
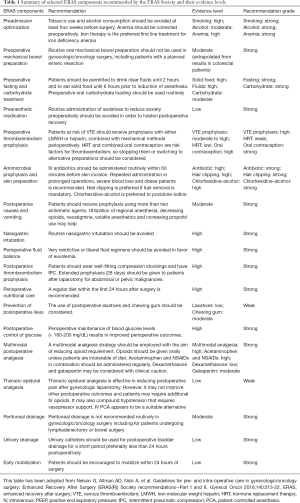
Full table
Herein we review the results that have been published in the literature to date regarding the ERAS protocols in ovarian cancer patients, and discuss why more evidence needs to be specifically assessed in this type of malignancy among other solid organ malignancies and even among other gynecologic cancers such as uterine and cervical cancers.
Individual components of ERAS
The ERAS guidelines for surgeries in gynecological oncology include about 20 elements that are recommended to follow before, during and after operations. We selected a number of individual elements that were thought to demand discussion in this review.
Preoperative fasting and carbohydrate loading
Patients who are planned to receive major abdominal surgery are usually restricted from eating solid food and drinking fluid for at least 8 hours conventionally. However, studies revealed that the intake of clear fluid until 2 hours before surgery does not increase gastric content, reduce gastric fluid pH, or increase complication rates (6,10). It has, therefore, been recommended to allow patients to take clear fluid until 2 hours and solid food until 6 hours before the induction of anesthesia unless the patient has conditions associated with gastroparesis such as previous surgery on esophagus, stomach or small intestine (10). Numerous studies have also shown that carbohydrate loading before surgery is associated with the attenuation of insulin resistance and early return of bowel function after surgery, proclaiming for its routine use in major abdominal surgery (11). For example, a randomized controlled study in colorectal cancer patients has demonstrated that the patients who received 100 g PrecarbTM (Vitaflo Limited, Liverpool, UK) dissolved in 800 mL of water the night before surgery and 50 g of VitajouleTM (Nestlé Health Science, Liverpool, UK) dissolved in 400 mL of water 3 hours prior to anesthesia showed a significantly earlier return of bowel function when compared to long fasting, thereby reducing the total length of hospital stay (12). Based on these data, increasing number of institutions have now adopted this element of perioperative care in their protocols for colorectal cancer patients. Despite the fact that these data were drawn from the patients who underwent bowel surgeries and not gynecologic surgeries, the ERAS Society concludes that these findings are valid for gynecologic patients as well given the similarities in patient characteristics (6,7). No studies have evaluated the effects of short fasting or carbohydrate loading on clinical outcomes in ovarian cancer patients yet. Even though it seems there is widespread adherence of short period of preoperative fasting and carbohydrate loading in colorectal surgery, study published in 2016 showed that a significant number of gynecologic institutions still maintained a fasting period more than 6 hours for fluids and more than 12 hours for solid food prior to surgery in gynecologic oncology (13). These disparities may be attributable to the lack of studies confirming the optimal duration of fasting as well as the role of preoperative carbohydrate loading in gynecological oncology in specific. Patients with ovarian cancer often have disseminated tumor lesions throughout the abdominal cavity, which often diminish bowel mobility. This particular disease characteristic should also be taken into account when designing future studies.
Preoperative bowel preparation
There are various methods of oral mechanical bowel preparations available to date (Table 2). Institutions often choose their methods based on surgeons’ preference, clinical feasibility and availability of specific agents. It is probably not an overestimation that a significant number of institutions have practiced this preoperative management prior to not only ovarian cancer surgeries but also other gynecologic oncology surgeries until recent. The ERAS gynecologic oncology guidelines, however, do not recommend routine oral mechanical bowel preparation before gynecologic oncology surgery (6,7). This even includes the patients who are planned to undergo bowel resection due to the involvement of tumor with gastrointestinal tract. The statements of the ERAS gynecologic oncology guidelines are based on a number of large clinical trials in which no increases in the rates of infection or anastomotic leakage were shown even after omitting mechanical bowel preparation before surgery (14). There has not been a definitive evidence of benefit but there are areas of concern with their use such as patient dissatisfaction, electrolyte disturbances and dehydration (15). However, studies that suggest doubtful views about omitting this procedure also exists, especially when the resecting regions include the rectum. In a single-blind, randomized controlled trial of rectal cancer patients undergoing low anterior resection, 178 patients were randomized to preoperative mechanical bowel preparation versus no preparation. Those patients in the mechanical bowel preparation group received senna solution (X-Prep sarget™) (1 or 2 120-mg packages of flavored powder diluted in a glass of water) 24 hours before the operation and 1 L of povidone-iodine enema on the evening before, and early in the morning (at least 2 hours) before surgery while the patients in the other group received none of these. The overall and infectious morbidity rates were significantly higher in no mechanical bowel preparation group versus mechanical bowel preparation group, 44% versus 27% (P=0.018), and 34% versus 16% (P=0.005), respectively. Regarding both anastomotic leakage and major morbidity rates, there was no significant difference between the two groups: 19% versus 10% (P=0.09) and 18% versus 11% (P=0.69), respectively. Mortality rates (1.1% vs. 3.4%) and mean hospital stay (16 vs. 14 days) did not differ significantly between the two groups. The authors concluded that surgeons should continue to perform preoperative mechanical bowel preparation before elective rectal resection due to the high risk of infectious morbidity seen in the study (16). It seems further studies are required to provide more solid evidence in order to omit mechanical bowel preparation in the preoperative setting particularly for the patients who are planned to receive low anterior resection in ovarian cancer surgery. Due to the anatomic proximity of the gynecologic organs with lower gastrointestinal tract such as sigmoid colon and rectum, it is not uncommon for ovarian cancer patients to receive bowel resection and anastomosis during their operation, most often undergoing low anterior resection. Therefore, with the lack of strong evidence to exclude mechanical bowel preparation before surgery, it may be imprudent for gynecologic surgeons to omit mechanical bowel preparation in patients who are likely to receive low anterior resection during their operations. Studies, indeed, report variations in the rates of adoption of this ERAS component (17). Bowel preparation are considered standard of care in Germany, whereas a Dutch single-center study reported that bowel preparation was avoided in 90% of gynecologic oncological procedures (13,18). In Australia and New Zealand, 55% of gynecologic oncologists have completely abandoned this procedure regardless of a planned bowel resection, including low anterior resection (19). High-quality studies assessing the consequences of omitting preoperative bowel preparation in the specific population of ovarian cancer patients are necessary to settle this issue.
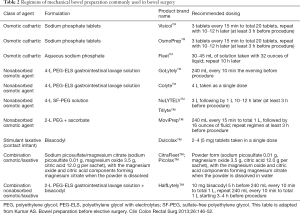
Full table
Prophylactic antibiotics
The ERAS gynecologic oncology guidelines recommend the administration of intravenous antibiotics within 60 minutes before skin incision (6). Most surgical interventions for ovarian malignancies include total hysterectomy. Total hysterectomy is classified as a clean-contaminated surgery, for which antibiotic prophylaxis before incision is recommended (20). Although there may be some variations, it seems that most gynecologic surgeons adhere to this guideline well. In one study in which the authors surveyed gynecologic surgeons in European nations, the second generation of cephalosporin was found to be used the most frequently, sometimes in combination with metronidazole (21). According to the study, prophylactic antibiotics were usually given as 1 g of cephalosporin diluted in 50 to 125 mL of saline solution infused for 15 minutes intravenously at anesthetic induction. Repeated intraoperative doses of antibiotics were also commonly used for prolonged operations, obese patients, and in cases with severe blood loss (21). Attributable to the solid evidence of its benefit and long standing of its use prior to surgical procedures, there seems to be less dissidents among gynecologic surgeons regarding the necessity of antimicrobial prophylaxis for ovarian cancer patients.
Early feeding
Early feeding is often defined as resuming oral fluid intake on the day of the operation and oral normal diet on the first postoperative day (18). A number of randomized controlled trials were conducted to assess this postoperative management in gynecologic surgeries (22,23). The studies consistently showed that early feeding was associated with significantly faster recovery of bowel function with shorter times to flatus and to tolerance of regular diet. The Cochrane review also states that early feeding in patients after major gynecologic surgery, excluding those who received bowel resection, hastens recovery of bowel function without increasing gastrointestinal morbidity (24). Although there are not as many studies assessing the role of early feeding in ovarian cancer patients as studies in other surgical disciplines, for example colorectal surgeries, one may extrapolate findings from surgical patients undergoing upper gastrointestinal procedures where early feeding seems to be safe and associated with shorter length of hospital stay (25). The ERAS gynecologic oncology guidelines advocate for a regular diet within the first 24 hours after operation (7). The Cochrane review also reiterates that early postoperative feeding is associated with early recovery of bowel function, lower rates of infectious complications, shorter length of hospital stay and higher patient satisfaction (24). Despite the strong recommendations of early feeding after surgery by numerous professional societies, a significant portion of gynecologic surgeons insist on the traditional postoperative care. According to one study, only 58% of gynecologic oncology patients were reported to be given a normal diet on the first postoperative day (18). Early feeding practices have been adopted widely in other surgical disciplines, but the implementation of this protocol in gynecologic oncology patients seems to be slow. In order to facilitate the adoption of this protocol in daily practice, the optimal feeding protocols in ovarian cancer patients need to be determined, specifically taking account of the wide range of surgical procedures performed in extensive cytoreductive surgery and prevalent malnutrition among ovarian cancer patients at diagnosis (26).
Perioperative fluid management
Optimizing fluid management is important for enhancing recovery after surgery (27). However, the optimal fluid management, hemodynamic parameters, and monitoring strategies to guide fluid management in ovarian cancer patients remain unclear. Evidence in colorectal surgery suggests that perioperative fluid overload delays the return of gastrointestinal function and prolongs hospital stay (28), whereas fluid restriction aiming at maintenance of body weight may reduce cardiopulmonary and wound complications (29). However, there are no specific guidelines regarding the optimal usage of fluid infusion perioperatively in ovarian cancer patients. Although the ERAS Society recently updated the perioperative fluid management section, which suggests the use of goal-directed fluid therapy, the heterogeneity in perioperative fluid management still remains in the current clinical settings (8). This suggests the needs for optimal regimen in ovarian cancer surgery to be determined. The variations in the scope of surgical procedures in ovarian malignancy differ from those seen in colorectal malignancy depending on patient’s tumor burden. Surgeries for early stages of ovarian cancer may take less than three hours to complete whereas advanced stage patients may have to undergo much extensive surgical procedures, often taking more than six hours to complete. This potential variation in stress response to surgery should also be considered when establishing guidelines for adequate perioperative fluid management in ovarian malignancy surgeries.
Postoperative analgesia
The optimal analgesic regimen for ovarian malignancy surgery has not been established yet and varies among institutions. Preemptive routine administration of epidural analgesia for patients undergoing major gynecologic oncology surgery is not currently recommended according to the ERAS guidelines (7). Epidural analgesia may be effective in reducing postoperative pain after gynecologic laparotomy. However, studies found that it may not improve other postoperative outcomes by hindering early ambulation, increasing urinary retention and increasing the risk of fluid overload (7). Epidural analgesia has also been shown to increase the length of hospital stay (30). Instead, utilizing multiple approaches to manage postoperative pain, such as by using oral medications combined with other methods of analgesia like spinal analgesia, use of non-opioid medications, continuous lidocaine infusions, or transversus abdominis plane blocks may be considered (31,32). The ERAS Society also recommends routine preoperative administration of oral acetaminophen, celecoxib, and gabapentin in its updated guidelines (8). It is difficult to determine the best combination of analgesics and the role of preemptive analgesia in ovarian cancer patients due to great discrepancies in patients’ responses to medications and extent of surgical procedures performed. Therefore, postoperative pain management should be tailored on individual bases with emphasis on providing adequate pain control, minimizing the use of opioids and facilitating early postoperative ambulation.
Avoidance of drains
Peritoneal drainage has traditionally been used in major abdominal surgery. It was thought to prevent accumulation of fluid in the bed of dissection, to evacuate blood, serous collections, and prevent anastomotic leakage (33,34). However, studies in rectal surgery found that peritoneal drainage does not prevent anastomotic leaks or improve overall outcome (35). Studies regarding drain uses after gynecologic oncology surgery are lacking. Nevertheless, the ERAS gynecologic oncology guidelines recommend the minimal use of drains and state specifically that there is no definitive evidence that drainage provides better clinical outcomes after gynecological surgery (7). This encompasses metastatic ovarian cancer surgery, in which the scope of surgery is larger often including the resection of other organs and peritoneal surfaces. The Cochrane systematic review also concludes that drains do not prevent the formation of lymphocysts, but are rather associated with a higher risk of cyst formation after pelvic lymphadenectomy (36). However, it may not be appropriate to extrapolate the results from colorectal cancer patients to gynecologic cancer patients. Unlike colorectal cancer patients, it is not uncommon to encounter ovarian cancer patients with a large amount of ascites at surgery. It is also common for them to undergo various extent of peritonectomy to remove disseminated tumor lesions, which may potentially decrease the amount of ascitic fluid reabsorption. Therefore, the role of postoperative peritoneal drain in ovarian cancer patients with ascitic fluid should further be assessed in future studies. It should also be investigated how peritoneal drainage serves as an indicator of postoperative bleeding because large sanguineous amount of drained fluid is oftentimes the first alarm for postoperative bleeding which clinicians can easily notice during postoperative care.
Avoidance of nasogastric decompression
Previous studies have shown the association of improved cardiovascular and respiratory complications with the avoidance of nasogastric tubes (37,38). Studies have also found that after early nasogastric tube removal, patients had significantly shorter time to return of bowel function and less subjective complaints, with no differences in time to tolerating normal diet and length of hospital stay (39). It has also been suggested that routine use of nasogastric decompression does not achieve reduction of postoperative paralytic ileus or overall earlier return of bowel function (40). Furthermore, meta-analyses that showed the increases in the risk of postoperative pneumonia after nasogastric intubation after elective abdominal surgery also support the avoidance of its use. (41). For above reasons, the ERAS guidelines suggest the avoidance of nasogastric tubes after gynecologic oncology surgery (8).
Postoperative chewing gum (prevention of postoperative ileus)
Despite the fact that many institutions offer postoperative laxatives in daily practice in order to facilitate early recovery of bowel function, no high-quality evidence exists to support its use in gynecologic oncology yet (7). However, the ERAS Society concludes that the use of laxatives is reasonable given the low cost and side effect profile despite the fact that data are limited and effects appear modest (7). They also cite a randomized controlled trial in which the researchers showed that postoperative chewing gum was associated with less incidence of postoperative ileus and reduced length of hospital stay in patients undergoing staging operations for gynecologic cancers (42). When 149 patients were randomly assigned to either chew sugar-free gum three times a day for 30 minutes each or to the control group, those who received chewing gum showed shorter time to first flatus (34.0±11.5 vs. 43.6±14.0 hours, P<0.001) and shorter time to first defecation (49.6±18.7 vs. 62.5±21.5, P<0.001). The two groups were comparable in terms of the complexity of surgical procedures performed. Based on these data, the ERAS protocols conclude that postoperative laxatives and chewing gum may be considered to prevent postoperative ileus and to hasten bowel function in gynecological oncology surgery.
Early removal of urinary drainage
The ERAS Society recommends the use of urinary catheters for less than 24 hours after gynecologic oncology surgery (7). Although there are no randomized controlled trials regarding the removal of urinary catheterization in ovarian cancer patients, studies suggest that early removal of catheterization are associated with less re-catheterization, less incidence of urinary tract infection and shorter hospital length of stay (43,44).
Prophylaxis for thromboembolism
Venous thromboembolism is a major risk in gynecologic oncology patients. Studies report the incidence rates as high as 38% in ovarian cancer patients (45). The ERAS gynecologic oncology guidelines recognize this high risk and recommend that all patients undergoing gynecologic oncologic surgery should receive venous thromboembolism prophylaxis. Specific recommendations include either low molecular weight heparin (LMWH) or heparin and that it should be provided before operation with mechanical thromboembolism prophylactic measures. The mechanical thromboembolism prophylactic measures include mobilization, graduated compression stockings (GCS), and intermittent pneumatic compression device (IPCD). These measures should be provided continuously throughout perioperative period (46,47). Pharmacologic prophylaxis regimens often used in the clinical settings include 5,000 units of unfractionated heparin given subcutaneously 2 hours prior to surgery and continued every 8–12 hours postoperatively, LMWH such as dalteparin 5,000 units given subcutaneously daily, and enoxaparin 40 mg given subcutaneously daily. Thromboembolism prophylaxis is certainly important in women undergoing surgery for advanced stage ovarian cancer and should be considered standard of care (13). Growing evidence supports the prolonged use of thromboembolic prophylaxis after cancer surgery as well (48).
Difficulties in applying ERAS in ovarian cancer
Ovarian cancer patients are in many ways distinctly different not only from other surgical patients but also from other gynecologic cancer patients. At the time of diagnosis, they often have advanced-stage disease with a high symptom burden including abdominal distension, dyspnea, nausea, impairment of gastrointestinal function, cachexia, and malnutrition (49). Due to the aggressive nature of the disease, the patients are often not eligible for minimally invasive techniques, and operative procedures can include a wide range of surgical procedures. It is also common for surgeons to make decisions whether to execute particular surgical procedures or not intraoperatively. In contrast to colorectal malignancies, in which palliative systemic treatment is the mainstay for advanced or metastatic disease, the initial choice of treatment for advanced ovarian cancer is cytoreductive surgery unless the patient has non-operable conditions. Therefore, the surgical complexity of ovarian malignancy often differs from that of colorectal malignancy. The patients who undergo extensive cytoreductive surgery with multivisceral resections are prone to be at a high risk of postoperative morbidity (9). These patients may also show excessive response to surgical stress with metabolic catabolism and systemic inflammatory response syndrome (9). Therefore, it may be inappropriate to directly apply the results regarding the ERAS protocols obtained from other surgical disciplines to patients with ovarian malignancies. The variable adoption rates of the ERAS protocols in ovarian cancer patients observed to date may be due to ongoing uncertainty about the applicability of evidence from either non-ovarian cancer surgery or less complex surgeries in gynecologic malignancies.
Strong beliefs in traditional surgical paradigm held by gynecologic surgeons also hinder the adoption of the ERAS protocols. Changing mind-sets of healthcare providers often require an intensive and well-planned approach (50,51). Although favorable evidence has been consistently provided by several prospective and retrospective studies, not many comprehensive randomized controlled trials evaluating the ERAS protocols have been conducted to provide strong evidence in gynecologic oncology (52). As the scope of surgical procedures become extensive, surgeons may naturally incline to adhere to the practices that have been performed for many years by their predecessors unless there is strong enough evidence to change their behavior.
Oncological outcomes with the ERAS
It is undeniably important to establish accurate assessments of what the implantation of ERAS protocols would bring to the current clinical settings in short-term. However, it is also crucial to consider the potential long-term effects of adopting the protocols, such as on patient survivals and disease-free survivals. No studies have been performed to evaluate the effects of the bundled ERAS protocols on oncologic outcomes in ovarian malignancy yet.
Among the individual components of the ERAS protocols, only one component has been studied to investigate its effects on oncologic outcomes. It is perioperative epidural analgesia that was shown to have a favorable effect in solid tumor malignancies. Researchers suggested that it may reduce surgical stress response experienced by patients and improve their immune function (53). Cell-mediated immunity that recognizes and responds to foreign antigens including tumor cells, plays a crucial role in preventing tumor metastasis (54). However, increased surgical stress response facilitates the release of cytokines and catecholamines that consequently inhibits cell-mediated immunity (55). Perioperative epidural use is thought to alleviate these surgical stress response (54). Studies on ovarian cancer surgery demonstrated this association between epidural anesthesia and improved progression-free survival and overall survival. In a retrospective study in which researchers reviewed 194 patients undergoing cytoreductive surgery for epithelial ovarian cancer, addition of epidural analgesia was found to be associated with a lower overall rate of recurrence compared with general anesthesia alone (72 vs. 85%, P=0.028) (56). Longer median progression-free survival was also associated with more than 48 hours of epidural use (14.9 months) compared with fewer than 24 hours (10.9 months) or 24–48 hours of epidural use (10.0 months; P=0.025) (56). Another study in which 648 patients who underwent primary debulking surgery for epithelial ovarian cancer were reviewed also demonstrated that epidural anesthesia was independently associated with a decreased risk of progression (HR =1.327; 95% CI, 1.066–1.653) and death (HR =1.588; 95% CI, 1.224–2.060) (55). However, despite the favorable effects of epidural analgesia on oncologic outcomes demonstrated in aforementioned studies, caution must be taken when interpreting these data. For example, a study showed that epidural analgesia may increase the length of hospital stay (30). The current standard treatment of epithelial ovarian cancer is primary cytoreductive surgery followed by platinum-based chemotherapy (57). It was reported that delays in the initiation of adjuvant chemotherapy were associated with decreased survival suggesting that recovering from surgery as early as possible in order to receive adjuvant chemotherapy is important (58-60). If the beneficial effects of epidural analgesia on oncological outcomes outweigh the potential increase of hospital stay and thereby delaying subsequent adjuvant treatment, the increase of hospital stay due to epidural analgesia may be excused. A further studies are required to evaluate the long-term effects of bundled ERAS protocols on oncologic outcomes in ovarian malignancies including this issue.
Patient compliance
Previous studies on the implementation of the ERAS protocols have shown that it is crucial to achieve patient compliance in order to accomplish optimal care (61). It was shown that clinical outcomes of the patients increase as the patient adherence to the given protocols increases (30). However, patient compliance is difficult to assess objectively. For example, if a patient who adhered to pre- and intraoperative ERAS protocols experiences surgical complications, thereby unable to follow the rest of the ERAS protocols postoperatively, it becomes difficult to determine whether the poor clinical outcome was caused by the surgical complications itself or the incompliance of the patient to the postoperative ERAS protocols. For this reason, some researchers excluded the ERAS elements of postoperative care when evaluating the association between compliance and patient outcomes (53). Pre- and intraoperative protocol elements are often under the control of the healthcare provider, whereas postoperative elements may be regarded as outcomes and dependent of the earlier elements (61). This also reflects that the care of surgical patients should be recognized as a continuum rather than a series of individual elements, since most of the elements influence those that follow (53). The successful adherence to one component of the ERAS protocols not only facilitate the adherence to another, but this intricate relations among each component allow synergistic improvement of patient recovery.
When researchers surveyed the practicing gynecologic oncologists in New Zealand and Australia, it was found that certain aspects of a clinical pathway of ERAS such as the prevention of hypothermia, thromboembolic prophylaxis, antimicrobial prophylaxis, and the avoidance of the routine use of drains were widely implemented in current practice. However, there was significant heterogeneity in preoperative fasting, postoperative feeding, postoperative pain management, fluid management, and prophylaxis of nausea and vomiting (19). Active and organized efforts to implement the ERAS protocols will not only increase patient adherence but also reduce the disparities in adopting individual components of the guidelines. Development and implementation of such protocols require multidisciplinary collaboration from all participating segments in patient care. This will enable the healthcare professionals to conglomerate individual components of ERAS protocols in service, which will consequently maximize patient compliance and outcomes. Benefits patients gain from the successful implementation of ERAS protocols in the care of ovarian cancer surgery seems promising. However, this requires a multidisciplinary team approach and resource-intensive implementation. An organized and systematic audit system is also a crucial part to examine compliance with each component of the ERAS protocols.
Future studies
It is undeniable that most studies about the implementation of the ERAS protocols in gynecological surgery published to date support the safety of the program (62-66). However, only few relevant published data are available on the ERAS protocols in ovarian cancer patients in specific. Given the nature of the interventions, it is impossible to blind participants and/or study investigators to the interventions. It may also be impossible to perform randomized controlled trials with bundled ERAS protocols due to the heterogeneity of the disease status of the patients. Furthermore, it may be ethically not feasible to try randomized trials of ERAS protocols given the positive evidence for ERAS elements reported to date. Well-designed cohort studies and novel trial designs may be alternatives to randomized controlled trials of individual interventions.
The current heterogeneity in patterns of care across and even within groups may challenge multicenter and international approach to perform a clinical trial. However, the fact that a growing number of ovarian cancer centers are gaining experience with ERAS protocols encourages international discussion and consensus. Efforts should be undertaken to coordinate the work of those institutions willing to revise their perioperative protocols, and cooperative trial groups could be a suitable platform to facilitate this process (21).
Conclusions
Consistent uptake of the ERAS principles in ovarian malignancies has been slow in comparison to other surgical disciplines, and not much data are available due to the lack of continuous quality improvements (67). The development and implementation of ERAS protocols in ovarian malignancies require a multidisciplinary approach of dedicated staff members committed to an ongoing process of monitoring and auditing with the aim of constant improvement of care. There is no doubt that the system-wide implementation of ERAS protocols can provide better patient outcomes in ovarian malignancies. However, in order to improve validity and generalizability of the protocols, current evidence seems insufficient and more studies of the protocols in the specific population of ovarian cancer patients are warranted. The ERAS protocols incorporate multiple interventions in patient care from preoperative preparations to postoperative discharge. Establishing solid evidence from well-designed clinical trials as well as continuously monitoring the adherence of the protocols are crucial to implement the ERAS protocols in clinical practice and maximize their impacts.
Acknowledgments
The authors are immensely grateful to Drs. Sang-Yoon Park and Jae-Weon Kim for sharing their pearls of wisdom during the course of this research and for providing comments on an earlier version of the manuscript. Any errors of this manuscript are our own and should not tarnish the reputations of them.
Funding: This work was supported by the National Research Foundation of Korea Grant, funded by the government of Republic of Korea (Ministry of Science and Information Technology) [2019R1F1A1063567].
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sang Yoon Park, Jae Weon Kim) for the series “Ultra-Radical Surgery in Ovarian Cancer: Surgical Techniques for Gynecologic Oncologist” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.04.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kehlet H. Fast-track colorectal surgery. Lancet 2008;371:791-3. [Crossref] [PubMed]
- Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763-4. [Crossref] [PubMed]
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921-8. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [Crossref] [PubMed]
- Gravante G, Elmussareh M. Enhanced recovery for non-colorectal surgery. World J Gastroenterol 2012;18:205-11. [Crossref] [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations--Part I. Gynecol Oncol 2016;140:313-22. [Crossref] [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations--Part II. Gynecol Oncol 2016;140:323-32. [Crossref] [PubMed]
- Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer 2019;29:651-68. [Crossref] [PubMed]
- Lindemann K, Kok PS, Stockler M, et al. Enhanced Recovery After Surgery for Advanced Ovarian Cancer: A Systematic Review of Interventions Trialed. Int J Gynecol Cancer 2017;27:1274-82. [Crossref] [PubMed]
- Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2011;28:556-69. [Crossref] [PubMed]
- Smith MD, McCall J, Plank L, et al. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 2014;CD009161 [Crossref] [PubMed]
- Noblett SE, Watson DS, Huong H, et al. Pre-operative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Colorectal Dis 2006;8:563-9. [Crossref] [PubMed]
- Muallem MZ, Dimitrova D, Pietzner K, et al. Implementation of Enhanced Recovery After Surgery (ERAS) Pathways in Gynecologic Oncology. A NOGGO-AGO* survey of 144 Gynecological Departments in Germany. Anticancer Res 2016;36:4227-32. [PubMed]
- Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;CD001544 [Crossref] [PubMed]
- Arnold A, Aitchison LP, Abbott J. Preoperative Mechanical Bowel Preparation for Abdominal, Laparoscopic, and Vaginal Surgery: A Systematic Review. J Minim Invasive Gynecol 2015;22:737-52. [Crossref] [PubMed]
- Bretagnol F, Panis Y, Rullier E, et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863-8. [Crossref] [PubMed]
- Forsmo HM, Pfeffer F, Rasdal A, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis 2016;18:603-11. [Crossref] [PubMed]
- de Groot JJ, van Es LE, Maessen JM, et al. Diffusion of Enhanced Recovery principles in gynecologic oncology surgery: is active implementation still necessary? Gynecol Oncol 2014;134:570-5. [Crossref] [PubMed]
- Lindemann K, Kok PS, Stockler M, et al. Enhanced Recovery After Surgery for Suspected Ovarian Malignancy: A Survey of Perioperative Practice Among Gynecologic Oncologists in Australia and New Zealand to Inform a Clinical Trial. Int J Gynecol Cancer 2017;27:1046-50. [Crossref] [PubMed]
- . ACOG Practice Bulletin No 195: Prevention of Infection After Gynecologic Procedures. Obstet Gynecol 2018;131:e172-89. [Crossref] [PubMed]
- Piovano E, Ferrero A, Zola P, et al. Clinical pathways of recovery after surgery for advanced ovarian/tubal/peritoneal cancer: an NSGO-MaNGO international survey in collaboration with AGO-a focus on surgical aspects. Int J Gynecol Cancer 2019;29:181-7. [Crossref] [PubMed]
- Kraus K, Fanning J. Prospective trial of early feeding and bowel stimulation after radical hysterectomy. Am J Obstet Gynecol 2000;182:996-8. [Crossref] [PubMed]
- Pearl ML, Frandina M, Mahler L, et al. A randomized controlled trial of a regular diet as the first meal in gynecologic oncology patients undergoing intraabdominal surgery. Obstet Gynecol 2002;100:230-4. [PubMed]
- Charoenkwan K, Matovinovic E. Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev 2014;CD004508 [Crossref] [PubMed]
- Willcutts KF, Chung MC, Erenberg CL, et al. Early Oral Feeding as Compared With Traditional Timing of Oral Feeding After Upper Gastrointestinal Surgery: A Systematic Review and Meta-analysis. Ann Surg 2016;264:54-63. [Crossref] [PubMed]
- Yim GW, Eoh KJ, Kim SW, et al. Malnutrition Identified by the Nutritional Risk Index and Poor Prognosis in Advanced Epithelial Ovarian Carcinoma. Nutr Cancer 2016;68:772-9. [Crossref] [PubMed]
- Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg 2006;202:971-89. [Crossref] [PubMed]
- Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002;359:1812-8. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg 2015;261:1153-9. [Crossref] [PubMed]
- Modesitt SC, Sarosiek BM, Trowbridge ER, et al. Enhanced Recovery Implementation in Major Gynecologic Surgeries: Effect of Care Standardization. Obstet Gynecol 2016;128:457-66. [Crossref] [PubMed]
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal Incision Injection of Liposomal Bupivacaine and Opioid Use After Laparotomy for Gynecologic Malignancies. Obstet Gynecol 2016;128:1009-17. [Crossref] [PubMed]
- Tsujinaka S, Kawamura YJ, Konishi F, et al. Pelvic drainage for anterior resection revisited: use of drains in anastomotic leaks. ANZ J Surg 2008;78:461-5. [Crossref] [PubMed]
- Averbach AM, Chang D, Koslowe P, et al. Anastomotic leak after double-stapled low colorectal resection. Dis Colon Rectum 1996;39:780-7. [Crossref] [PubMed]
- Petrowsky H, Demartines N, Rousson V, et al. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg 2004;240:1074-84; discussion 1084-5. [Crossref] [PubMed]
- Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in patients with gynaecological malignancies. Cochrane Database Syst Rev 2014;CD007387 [Crossref] [PubMed]
- Teeuwen PH, Bleichrodt RP, Strik C, et al. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointest Surg 2010;14:88-95. [Crossref] [PubMed]
- Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531-41. [Crossref] [PubMed]
- Pearl ML, Valea FA, Fischer M, et al. A randomized controlled trial of postoperative nasogastric tube decompression in gynecologic oncology patients undergoing intra-abdominal surgery. Obstet Gynecol 1996;88:399-402. [Crossref] [PubMed]
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007;CD004929 [PubMed]
- Cheatham ML, Chapman WC, Key SP, et al. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg 1995;221:469-76; discussion 476-8. [Crossref] [PubMed]
- Ertas IE, Gungorduk K, Ozdemir A, et al. Influence of gum chewing on postoperative bowel activity after complete staging surgery for gynecological malignancies: a randomized controlled trial. Gynecol Oncol 2013;131:118-22. [Crossref] [PubMed]
- Griffiths R, Fernandez R. Policies for the removal of short-term indwelling urethral catheters. Cochrane Database Syst Rev 2005;CD004011 [Crossref] [PubMed]
- Ahmed MR, Sayed Ahmed WA, Atwa KA, et al. Timing of urinary catheter removal after uncomplicated total abdominal hysterectomy: a prospective randomized trial. Eur J Obstet Gynecol Reprod Biol 2014;176:60-3. [Crossref] [PubMed]
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285-91. [Crossref] [PubMed]
- Baykal C, Al A, Demirtas E, et al. Comparison of enoxaparin and standard heparin in gynaecologic oncologic surgery: a randomised prospective double-blind clinical study. Eur J Gynaecol Oncol 2001;22:127-30. [PubMed]
- Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189-204. [Crossref] [PubMed]
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975-80. [Crossref] [PubMed]
- Chase DM, Wenzel L. Health-related quality of life in ovarian cancer patients and its impact on clinical management. Expert Rev Pharmacoecon Outcomes Res 2011;11:421-31. [Crossref] [PubMed]
- Grimshaw JM, Eccles MP, Walker AE, et al. Changing physicians' behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof 2002;22:237-43. [Crossref] [PubMed]
- Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet 2003;362:1225-30. [Crossref] [PubMed]
- Lu D, Wang X, Shi G. Perioperative enhanced recovery programmes for gynaecological cancer patients. Cochrane Database Syst Rev 2015;CD008239 [Crossref] [PubMed]
- Wijk L, Udumyan R, Pache B, et al. International validation of Enhanced Recovery After Surgery Society guidelines on enhanced recovery for gynecologic surgery. Am J Obstet Gynecol 2019;221:237.e1-237.e11. [Crossref] [PubMed]
- Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol 2001;2:293-9. [Crossref] [PubMed]
- Tseng JH, Cowan RA, Afonso AM, et al. Perioperative epidural use and survival outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer. Gynecol Oncol 2018;151:287-93. [Crossref] [PubMed]
- Elias KM, Kang S, Liu X, et al. Anesthetic selection and disease-free survival following optimal primary cytoreductive surgery for stage III epithelial ovarian cancer. Ann Surg Oncol 2015;22:1341-8. [Crossref] [PubMed]
- du Bois A, Quinn M, Thigpen T, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol 2005;16 Suppl 8:viii7-viii12.
- Kim BJ, Caudle AS, Gottumukkala V, et al. The Impact of Postoperative Complications on a Timely Return to Intended Oncologic Therapy (RIOT): the Role of Enhanced Recovery in the Cancer Journey. Int Anesthesiol Clin 2016;54:e33-46. [Crossref] [PubMed]
- Liu Y, Zhang T, Wu Q, et al. Relationship between initiation time of adjuvant chemotherapy and survival in ovarian cancer patients: a dose-response meta-analysis of cohort studies. Sci Rep 2017;7:9461. [Crossref] [PubMed]
- Lee YY, Lee JW, Lu L, et al. Impact of interval from primary cytoreductive surgery to initiation of adjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Gynaecol Obstet 2018;143:325-32. [Crossref] [PubMed]
- Gustafsson UO, Hausel J, Thorell A, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011;146:571-7. [Crossref] [PubMed]
- Gadducci A, Cosio S, Spirito N, et al. The perioperative management of patients with gynaecological cancer undergoing major surgery: A debated clinical challenge. Crit Rev Oncol Hematol 2010;73:126-40. [Crossref] [PubMed]
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol 2013;122:319-28. [Crossref] [PubMed]
- Minig L, Biffi R, Zanagnolo V, et al. Reduction of postoperative complication rate with the use of early oral feeding in gynecologic oncologic patients undergoing a major surgery: a randomized controlled trial. Ann Surg Oncol 2009;16:3101-10. [Crossref] [PubMed]
- Carter J. Fast-track surgery in gynaecology and gynaecologic oncology: a review of a rolling clinical audit. ISRN Surg 2012;2012:368014 [Crossref] [PubMed]
- Chase DM, Lopez S, Nguyen C, et al. A clinical pathway for postoperative management and early patient discharge: does it work in gynecologic oncology? Am J Obstet Gynecol 2008;199:541.e1-7. [Crossref] [PubMed]
- Nelson G, Dowdy SC, Lasala J, et al. Enhanced recovery after surgery (ERAS(R)) in gynecologic oncology - Practical considerations for program development. Gynecol Oncol 2017;147:617-20. [Crossref] [PubMed]


