
Combined deep inferior epigastric artery perforator flap with vascularized groin lymph node transplant for treatment of breast cancer-related lymphedema
Background
Around 3.5 million women in the United States are survivors of breast cancer, with almost 270,000 new cases diagnosed annually (1). Breast cancer-related lymphedema (BCRL) remains the greatest cancer survivorship burden for breast cancer survivors (2-5), affecting around 30% of patients treated for breast cancer (6), and all survivors remain at risk of developing clinical lymphedema over their lifetime (7). Modern surgical techniques have demonstrated effectiveness at decreasing the symptoms of lymphedema, reducing the risk of future infections, decreasing the amount of time spent daily for lymphedema care and improving quality of life (8-13). These procedures can be broadly categorized as physiological or debulking. Physiological procedures, including lymphovenous bypass (LVB) and vascularized lymph node transplant (VLNT), aim to restore lymphatic fluid drainage within the affected extremity (14-19). The VLNT procedure is indicated in advanced stage lymphedema and involves microvascular anastomosis to perfuse and maintain function of the lymph nodes transplanted into an extremity, either to an anatomical (orthotopic) or non-anatomical (heterotopic) location, to restore physiological lymphatic function (9,10,14-17). Orthotopic VLNT has the additional advantage of the opportunity for radical axillary scar release/lysis to allow drainage from the affected extremity, relieve restrictions to upper extremity range of motion, create a recipient bed for the lymph node flap, and in cases of venous insufficiency decompress the axillary/subclavian vein.
Vascularized lymph node flaps may be harvested from within the inguinal, axillary, or cervical lymph node basins, as well as from within the abdomen; these include the superficial inguinal (groin) (20,21), lateral thoracic (22-25), supraclavicular (26-29), submental (30), omental (gastroepiploic) (31-33), and jejunal mesenteric flaps (34,35). The groin lymph node flap, which incorporates lymph nodes from within the superficial inguinal lymph node basin, remains the most commonly performed VLNT due to the well-understood vascular anatomy and consistent lymph node yield (36-39). In patients undergoing postmastectomy breast reconstruction the superficial inguinal (groin) vascularized lymph node flap can be transferred en bloc with a deep inferior epigastric artery perforator (DIEP) or muscle-sparing transverse rectus abdominis myocutaneous (MS-TRAM) flap in a single operation without the need for additional scars (10,37,38). This article reviews the indications, preoperative assessment, surgical technique, complications, and lessons learned for this procedure.
Indications/contraindications
Combined DIEP flap and groin VLNT is indicated in patients with postmastectomy lymphedema that desire/require microvascular autologous flap breast reconstruction. These patients have typically received postmastectomy radiation therapy (PMRT) and therefore autologous reconstruction is generally favored over implant-based reconstruction; optimal timing for breast reconstruction is typically after 6 months following completion of radiation therapy (40). In patients with significant axillary scarring that is limiting upper extremity mobility, and where it is contributing to venous insufficiency, there is an opportunity for radical axillary scar release with decompression of the axillary vein by removing extrinsic scar. Where the forearm/hand are affected, the authors routinely combine DIEP flap/groin VLNT with LVB in the forearm which have a synergistic effect on treating the lymphedema, with the two procedures working to complement each other by different mechanisms.
Our indications for performing these procedures with respect to body mass index (BMI) are similar to those of autologous breast reconstruction, although lymphedema may be precipitated spontaneously at extremes of BMI (41). Obese patients are often the patient group most severely affected by lymphedema and potentially have the most to gain by these surgeries, although they must be counselled regarding the higher rate of wound-related complications (42).
Absolute contraindications include severe medical comorbidities, unresectable chest wall disease, uncontrolled metastatic disease, and specifically prior ligation of the deep inferior epigastric (DIE) pedicle or prior abdominoplasty. Relative contraindications include active cigarette smoking and the presence of multiple abdominal scars (43). If a prior pfannenstiel incision transgresses between the abdominal flap and groin lymph node flap then additional anastomosis of both the superficial circumflex iliac artery (SCIA) and vein (SCIV) is required. Patients with history suggestive of thrombophilia undergoing any microvascular flap procedure should undergo preoperative hematological testing.
Preoperative assessment
Patients undergo assessment of their lymphedema preoperatively, which includes clinical examination, limb volume assessment using a perometer, and bioimpedance spectroscopy, as well as staging investigations including radionuclide lymphoscintigraphy or near-infrared fluorescent lymphatic imaging (NIRFLI). Duplex ultrasonography is indicated to exclude venous thrombosis and venous insufficiency. Preoperative imaging using computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) for perforator/pedicle selection is important for microvascular abdominal flap breast reconstruction, especially where there are multiple abdominal scars or a history of abdominal liposuction, and also for evaluation of the groin lymph node flap vascular anatomy and lymph node localizations.
A rare but devastating disadvantage of harvest of lymph node flaps from the inguinal region is the potential to cause donor site lower extremity lymphedema; we recommend that reverse lymphatic mapping using sentinel lymph node biopsy techniques is always used to enable intraoperative guidance to avoid lymph nodes within a regional lymphatic basin that drain the donor extremity to avoid the risk of causing iatrogenic leg lymphedema (22). Preoperative radionuclide lymphoscintigraphy can also be performed the day prior to surgery with the use of single-photon emission CT (SPECT) imaging to provide multiplanar visualization of the sentinel lymph node locations with the inguinal region to allow presurgical planning as well as intraoperative guidance using a gamma detection probe system (Figure 1).
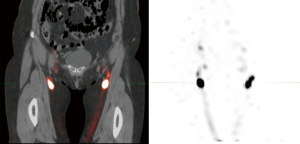
Surgical technique
The dominant blood supply to the transverse lower abdominal tissue is from perforators of the DIE pedicle, a branch of the external iliac vessels, via the rectus abdominis muscle. This is the main vascular supply to the microvascular transverse abdominal flap, harvested as either a DIEP or MS-TRAM flap. Although there is a variable contribution from the superficial inferior epigastric artery and vein (SIEA/SIEV), perfusion across the midline is unreliable, and in practice the SIE vessels are only used as a secondary blood supply in bipedicled flap designs (38). The superficial inguinal (groin) lymph node basin that drains the lower abdomen supplied by the SCIA/SCIV is the target for lymph node flap harvest from this region. Flap markings are similar to a standard free abdominal flap/abdominoplasty and the preferred side for lymph node flap harvest can be determined from preoperative imaging.
First, staging is performed intraoperatively using indocyanine green (ICG) NIRFLI. Intradermal injection of ICG into the webspaces of the affected upper extremity allows the lymphatic vessels to be mapped. LVB can be performed to any obstructed lymphatic vessels visualized, and targeted supermicrosurgical bypass of these are performed to neighboring venules using standard techniques (44).
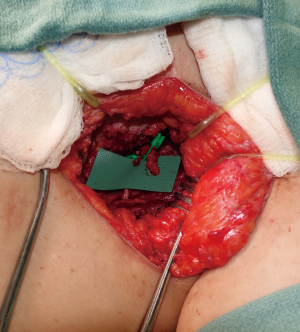
Radical axillary scar release is performed to create a recipient bed for the lymph node flap and expose branches of the subscapular axis for anastomosis of the lymph node flap; typically a branch of the thoracodorsal vessels or the serratus vessels are used as recipient vessels, preserving the latissimus dorsi flap lifeboat (Figure 2). We advocate that care must be taken to lyse the perivascular scar around the recipient vessels to prevent flap venous congestion, as well as any bands compressing the axillary/subclavian vein which can provide some independent improvement in the lymphedema.
Detailed knowledge of the lymphovascular anatomy and variability of the superficial and deep inguinal lymph node flap donor site is essential to avoid the risk of iatrogenic donor-site lower extremity lymphedema and ensure viability of the lymph nodes within the flap. The superficial inguinal lymph nodes are separated by distinct fascial boundaries from the deep lymph node basins adjacent to the femoral vessels that drain the lower extremity (20,21,36). Intradermal injection of ICG at multiple locations cephalad to the lower abdominal incision can facilitate identification of the superficial inguinal lymph nodes using intraoperative fluorescent imaging guidance and ensure that these are included within the flap (22).
The inferior incision is made first to raise the lymph node flap en bloc with the abdominal flap. Flap harvest is performed at the suprafascial (Scarpas) plane with care to include the SIEA/SIEV and SCIA/SCIV to ensure perfusion (36-39,45,46). Dissection medial to the lateral border of femoral artery, caudal to the groin crease, and deep to the fascia of the thigh, must be avoided (47-49) (Figure 3). The lymph node flap is raised under gamma probe reverse lymphatic mapping guidance to avoid the sentinel lymph nodes draining the lower extremity.
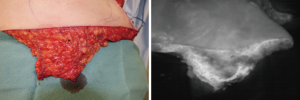
Next, we proceed to harvest of the microvascular abdominal flap. If there is one dominant perforator, or two or three suitable perforators in the same intramuscular septum, then a DIEP flap is raised; if not then as much muscle as is necessary to incorporate multiple smaller perforators is included for a MS-TRAM flap. As the flap needs to reach the apex of the axilla, dissection of the DIE pedicle is performed to the origin at the external iliac vessels to maximize the pedicle length; we typically prefer anastomosis to the internal mammary recipient vessels.
Where the lymph node flap is located on the hemiabdomen ipsilateral to the perforators supplying the flap or where bilateral flap reconstruction is performed, additional anastomosis of the SCIV to a vein from the subscapular axis in the axilla may be required to adequately drain the lymph nodes; we have found the use of intraoperative fluorescent perfusion clearance imaging to be very helpful in aiding this decision (Figure 3). Where the lymph node flap is located on the contralateral hemiabdomen to the perforators, additional anastomosis of both the SCIA and SCIV to branches of the subscapular system within the axilla is necessary to perfuse the lymph node flap. The presence of the SCIA is variable, and where absent a bipedicled flap design is required to ensure perfusion of the lymph node flap; the SCIA pedicle length is typically short and the arterial caliber is usually small (50). Care should be taken to close the donor site to avoid a contour defect postoperatively, and advancement of the superior abdominal flap can be helpful to reconstruct the volume defect (51).
Postoperative flap monitoring is performed using a standard free flap protocol with the patient initially cared for flexed at the abdomen; inpatient stay is typically 3–4 days. Perioperative antibiotics are instituted to reduce the risk of upper extremity cellulitis. Compression garment use is recommenced at 2–4 weeks postoperatively and strenuous activities are avoided for 6–8 weeks.
Complications
Total flap failure rate is typically around 1–2% for free DIEP or MS-TRAM flaps (52). Other complications include venous congestion, hematoma, partial flap loss, fat necrosis, seroma, wound infection or breakdown, and donor-site bulge or hernia; obesity and smoking are associated with surgical site complications (43). Abdominal donor site surgical drains typically need to remain in situ for longer when groin lymph nodes are harvested due to higher drain output.
Outcomes
Studies have reported favorable outcomes for lymphedema treatment using combined microvascular abdominal flap breast reconstruction with groin VLNT. Saaristo et al. evaluated outcomes in 9 patients with upper extremity BCRL that underwent microvascular abdominal flap breast reconstruction with superficial inguinal VLNT and found that limb circumference was reduced in 7 of 9 patients (78%), and compression therapy was no longer required in 3 patients (33%); in patients that underwent postoperative lymphoscintigraphy, improvement in lymphatic vessel function was found in 5 of 6 patients (83%) (37). In another study, Nguyen and colleagues evaluated outcomes in 29 patients with lymphedema that underwent microvascular abdominal flap breast reconstruction combined with groin VLNT and found that sustained improvement in symptoms of lymphedema were reported in 23 of 29 patients (79%) at a mean follow-up of 11 months (range, 3–33 months) after reconstruction (38); mean differential volumes measured using a perometer in all of the patients improved from 21% increased volumes preoperatively to 20%, 19%, 14%, and 10% at 1, 3, 6, and 12 months after reconstruction, respectively. De Brucker et al. evaluated outcomes in 25 patients with BCRL, of which 22 underwent simultaneous DIEP flap with groin VLNT, and 2 underwent microvascular groin VLNT to the axilla following prior DIEP flap breast reconstruction (53). Eleven patients (44%) discontinued compression therapy postoperatively. Of 6 patients with recurrent infections, 3 did not experience a further infection and in the other 3 the frequency was reduced with mean follow-up of 29 months (range, 8–64 months); patient reported outcomes scores using the Upper Limb Lymphedema-27 questionnaire were significantly improved postoperatively, with improved questionnaire scores in 21 of 25 patients (84%).
Discussion
The combined DIEP flap with groin VLNT procedure is indicated in patients with postmastectomy BCRL undergoing microvascular autologous flap breast reconstruction. Autologous breast reconstruction is typically indicated in patients that have received PMRT, which is an independent risk factor for the development of lymphedema (3-5,54). The advantages of combining microvascular abdominal flap reconstruction with groin VLNT include achieving breast reconstruction and treatment of lymphedema potentially in a single operation, optimization of the blood supply to the lymph node flap via the abdominal flap carrier given the anatomical variability of the SCIA, avoidance of an additional scar for lymph node flap harvest, and opportunity for axillary scar release. Whether performing this surgery in patients at high risk of developing lymphedema can prevent its occurrence is currently under investigation.
The decision regarding placement of the VLNT to an orthotopic or heterotopic location within the affected extremity remains a subject of debate (55-62). Experimental and clinical studies have demonstrated that orthotopic VLNT functions by a “bridging” mechanism via lymphangiogenesis with new afferent and efferent lymphatic collateral pathways connecting the transplanted lymph nodes with lymphatic vessels in the recipient site to restore outflow (55,63,64). Where the distribution of the lymphedema also affects the forearm/hand we have found that performing LVB to the forearm provides synergistic improvement of the lymphedema, with VLNT and LVB functioning by different mechanisms.
A rare but devastating risk of harvest of lymph node flaps from the inguinal region is the potential to cause donor extremity lymphedema (47,65,66). In one prospective study donor lower extremity lymphedema was diagnosed in 2 of 14 patients following groin VLNT (16 flaps) to treat upper extremity lymphedema (47), and in another series lower limb swelling after groin lymph node flap harvest was reported in 1 of 42 patients (66). There is also evidence that even in the absence of clinical lower extremity lymphedema lymphatic function may still be compromised: in one study of 30 consecutive patients that underwent groin VLNT found that at a mean of 13 months after surgery, the transport indexes were significantly higher in the donor limb but within the normal range, with no lymphedema clinically or on lymphoscintigraphy (67); in another study postoperative lymphoscintigraphy was performed in 10 patients who underwent groin VLN flap harvest and minor changes in lymphatic flow were noted in 6 patients, with an abnormal transport index in 2 patients, but without clinical evidence of lymphedema (68). The same group found an abnormal lymphatic transport index on lymphoscintigraphy in 2 of 13 patients, but in none out of 16 cases where a modified groin lymph node flap harvest technique was used. This modified technique included harvest of only the SCIA, no tissue medial to the femoral artery in order to avoid the sentinel lymph nodes, and inclusion of only one palpable lymph node within the flap (69). Detailed knowledge of the lymphovascular anatomy and variability of the inguinal donor site is therefore essential to avoid the risk of iatrogenic donor site lower extremity lymphedema. The authors recommend that dissection medial to the lateral border of femoral artery, caudal to the groin crease, and deep to the fascia of the thigh, is absolutely avoided (47-49). We also always use preoperative SPECT reverse lymphatic mapping using radioactive isotope to identify the sentinel lymph nodes draining the lower limb so that they can be preserved during surgery by observing counts using a gamma probe for groin vascularized lymph node flap harvest, and we believe this to be essential to avoid the risk of donor extremity lymphedema given individual lymphatic anatomical variations (48).
Conclusions
Groin VLNT combined with microvascular DIEP flap breast reconstruction allows for treatment of lymphedema and autologous breast reconstruction in one operation and without additional scars or hospital stay. Combining this procedure with LVB if indicated can have synergistic benefit for treating lymphedema affecting the forearm/hand. Although a risk, symptomatic iatrogenic donor site lymphedema is a rare occurrence that can be minimized provided that surgeons strictly adhere to flap harvest guidelines to limiting the extent of the dissection and always employ reverse lymphatic mapping.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Charles E Butler, Carrie Chu, and Margaret Roubaud) for the series “New Frontiers in Breast Reconstruction” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.02.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Available online: https://seer.cancer.gov
- Cormier JN, Askew RL, Mungovan KS, et al. Lymphedema beyond breast cancer. Cancer 2010;116:5138-49. [Crossref] [PubMed]
- Voss RK, Cromwell KD, Chiang YJ, et al. The long-term risk of upper-extremity lymphedema is two-fold higher in breast cancer patients than in melanoma patients. J Surg Oncol 2015;112:834-40. [Crossref] [PubMed]
- Nguyen TT, Hoskin TL, Habermann EB, et al. Breast Cancer-Related Lymphedema Risk is Related to Multidisciplinary Treatment and Not Surgery Alone: Results from a Large Cohort Study. Ann Surg Oncol 2017;24:2972-80. [Crossref] [PubMed]
- Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer 2018;25:309-14. [Crossref] [PubMed]
- Miller CL, Specht MC, Skolny MN, et al. Risk of lymphedema after mastectomy: potential benefit of applying ACOSOG Z0011 protocol to mastectomy patients. Breast Cancer Res Treat 2014;144:71-7. [Crossref] [PubMed]
- Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology 2010;43:118-27. [PubMed]
- Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017;37:947-53. [Crossref] [PubMed]
- Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73-9. [Crossref] [PubMed]
- Gratzon A, Schultz J, Secrest K, et al. Clinical and Psychosocial Outcomes of Vascularized Lymph Node Transfer for the Treatment of Upper Extremity Lymphedema After Breast Cancer Therapy. Ann Surg Oncol 2017;24:1475-81. [Crossref] [PubMed]
- Ozturk CN, Ozturk C, Glasgow M, et al. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J Plast Reconstr Aesthet Surg 2016;69:1234-47. [Crossref] [PubMed]
- Hoffner M, Bagheri S, Hansson E, et al. SF-36 Shows Increased Quality of Life Following Complete Reduction of Postmastectomy Lymphedema with Liposuction. Lymphat Res Biol 2017;15:87-98. [Crossref] [PubMed]
- Sharkey AR, King SW, Ramsden AJ, et al. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency? Microsurgery 2017;37:348-53. [Crossref] [PubMed]
- Chang DW, Masia J, Garza R 3rd, et al. Lymphedema: Surgical and Medical Therapy. Plast Reconstr Surg 2016;138:209S-218S. [Crossref] [PubMed]
- Schaverien MV, Coroneos CJ. Surgical Treatment of Lymphedema. Plast Reconstr Surg 2019;144:738-58. [Crossref] [PubMed]
- Silva AK, Chang DW. Vascularized lymph node transfer and lymphovenous bypass: Novel treatment strategies for symptomatic lymphedema. J Surg Oncol 2016;113:932-9. [Crossref] [PubMed]
- Suami H, Chang DW. Overview of surgical treatments for breast cancer-related lymphedema. Plast Reconstr Surg 2010;126:1853-63. [Crossref] [PubMed]
- Schaverien MV, Badash I, Patel KM, et al. Vascularized Lymph Node Transfer for Lymphedema. Semin Plast Surg 2018;32:28-35. [Crossref] [PubMed]
- Chang EI, Masià J, Smith ML. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin Plast Surg 2018;32:36-41. [Crossref] [PubMed]
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98. [Crossref] [PubMed]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Barreiro GC, Baptista RR, Kasai KE, et al. Lymph fasciocutaneous lateral thoracic artery flap: Anatomical study and clinical use. J Reconstr Microsurg 2014;30:389-96. [Crossref] [PubMed]
- Tinhofer IE, Meng S, Steinbacher J, et al. The surgical anatomy of the vascularized lateral thoracic artery lymph node flap: A cadaver study. J Surg Oncol 2017;116:1062-8. [Crossref] [PubMed]
- Smith ML, Molina BJ, Dayan E, et al. Heterotopic vascularized lymph node transfer to the medial calf without a skin paddle for restoration of lymphatic function: Proof of concept. J Surg Oncol 2017;115:90-5. [Crossref] [PubMed]
- Akita S, Mitsukawa N, Kuriyama M, et al. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann Plast Surg 2015;74:573-9. [Crossref] [PubMed]
- Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J Surg Oncol 2017;115:68-71. [Crossref] [PubMed]
- Sapountzis S, Singhal D, Rashid A, et al. Lymph node flap based on the right transverse cervical artery as a donor site for lymph node transfer. Ann Plast Surg 2014;73:398-401. [Crossref] [PubMed]
- Steinbacher J, Tinhofer IE, Meng S, et al. The surgical anatomy of the supraclavicular lymph node flap: A basis for the free vascularized lymph node transfer. J Surg Oncol 2017;115:60-2. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Huang JJ, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93-8. [Crossref] [PubMed]
- Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: Technique and outcomes. Microsurgery 2017;37:197-205. [Crossref] [PubMed]
- Ciudad P, Agko M, Perez Coca JJ, et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J Surg Oncol 2017;116:671-82. [Crossref] [PubMed]
- Nguyen AT, Suami H, Hanasono MM, et al. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017;115:84-9. [Crossref] [PubMed]
- Coriddi M, Wee C, Meyerson J, et al. Vascularized Jejunal Mesenteric Lymph Node Transfer: A Novel Surgical Treatment for Extremity Lymphedema. J Am Coll Surg 2017;225:650-7. [Crossref] [PubMed]
- Schaverien MV, Hofstetter WL, Selber JC. Vascularized Jejunal Mesenteric Lymph Node Transfer for Lymphedema: A Novel Approach. Plast Reconstr Surg 2018;141:468e-469e. [Crossref] [PubMed]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Akita S, Tokumoto H, Yamaji Y, et al. Contribution of simultaneous breast reconstruction by deep inferior epigastric artery perforator flap to the efficacy of vascularized lymph node transfer in patients with breast cancer-related lymphedema. J Reconstr Microsurg 2017;33:571-8. [Crossref] [PubMed]
- Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg 2011;127:1100-6. [Crossref] [PubMed]
- Greene AK. Diagnosis and Management of Obesity-Induced Lymphedema. Plast Reconstr Surg 2016;138:111e-118e. [Crossref] [PubMed]
- Chang EI, Liu J. Prospective Evaluation of Obese Patients Undergoing Autologous Abdominal Free Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:120e-125e. [Crossref] [PubMed]
- Fischer JP, Wes AM, Tuggle CT, et al. Risk analysis and stratification of surgical morbidity after immediate breast reconstruction. J Am Coll Surg 2013;217:780-7. [Crossref] [PubMed]
- Chang EI, Skoracki RJ, Chang DW. Lymphovenous Anastomosis Bypass Surgery. Semin Plast Surg 2018;32:22-7. [Crossref] [PubMed]
- Chen R, Mu L, Zhang H, et al. Simultaneous breast reconstruction and treatment of breast cancer-related upper arm lymphedema with lymphatic lower abdominal flap. Ann Plast Surg 2014;73:S12-S17. [Crossref] [PubMed]
- Tourani SS, Taylor GI, Ashton MW. Scarpa fascia preservation in abdominoplasty: Does it preserve the lymphatics? Plast Reconstr Surg 2015;136:258-62. [Crossref] [PubMed]
- Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516-20. [Crossref] [PubMed]
- Scaglioni MF, Suami H. Lymphatic anatomy of the inguinal region in aid of vascularized lymph node flap harvesting. J Plast Reconstr Aesthet Surg 2015;68:419-27. [Crossref] [PubMed]
- Dayan JH, Dayan E, Kagen A, et al. The use of magnetic resonance angiography in vascularized groin lymph node transfer: An anatomic study. J Reconstr Microsurg 2014;30:41-5. [Crossref] [PubMed]
- Gharb BB, Rampazzo A, Spanio di Spilimbergo S, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg 2011;67:589-93. [Crossref] [PubMed]
- Maldonado AA, Garza RM, Artz J, et al. Abdominal flap for closing the donor site after groin lymph node transfer. J Surg Oncol 2017;115:390-1. [Crossref] [PubMed]
- Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive Evaluation of Risk Factors and Management of Impending Flap Loss in 2138 Breast Free Flaps. Ann Plast Surg 2016;77:67-71. [Crossref] [PubMed]
- De Brucker B, Zeltzer A, Seidenstuecker K, et al. Breast Cancer-Related Lymphedema: Quality of Life after Lymph Node Transfer. Plast Reconstr Surg 2016;137:1673-80. [Crossref] [PubMed]
- Shaitelman SF, Chiang YJ, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: a systematic review and network meta-analysis. Breast Cancer Res Treat 2017;162:201-15. [Crossref] [PubMed]
- Shesol BF, Nakashima R, Alavi A, et al. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg 1979;63:817-23. [Crossref] [PubMed]
- Chen HC, O’Brien BM, Rogers IW, Pribaz JJ, Eaton CJ. Lymph node transfer for the treatment of obstructive lymphedema in the canine model. Br J Plast Surg 1990;43:578-86. [Crossref] [PubMed]
- Patel KM, Lin CY, Cheng MH. From theory to evidence: Long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg 2015;31:26-30. [Crossref] [PubMed]
- Suami H, Scaglioni MF, Dixon KA, et al. Interaction between vascularized lymph node transfer and recipient lymphatics after lymph node dissection: A pilot study in a canine model. J Surg Res 2016;204:418-27. [Crossref] [PubMed]
- Ito R, Zelken J, Yang CY, et al. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol 2016;141:182-8. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: Natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192e-198e. [Crossref] [PubMed]
- Yan A, Avraham T, Zampell JC, et al. Mechanisms of lymphatic regeneration after tissue transfer. PLoS One 2011;6:e17201. [Crossref] [PubMed]
- Aschen SZ, Farias-Eisner G, Cuzzone DA, et al. Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast Reconstr Surg 2014;133:301-10. [Crossref] [PubMed]
- Viitanen TP, Visuri MT, Hartiala P, et al. Lymphatic vessel function and lymphatic growth factor secretion after microvascular lymph node transfer in lymphedema patients. Plast Reconstr Surg Glob Open 2013;1:1-9. [Crossref] [PubMed]
- Huang JJ, Gardenier JC, Hespe GE, et al. Lymph node transplantation decreases swelling and restores immune responses in a transgenic model of lymphedema. PLoS One 2016;11:e0168259. [Crossref] [PubMed]
- Demiri E, Dionyssiou D, Tsimponis A, et al. Donor-site lymphedema following lymph node transfer for breast cancer-related lymphedema: a systematic review of the literature. Lymphat Res Biol 2018;16:2-8. [Crossref] [PubMed]
- Pons G, Masia J, Loschi P, et al. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J Plast Reconstr Aesthet Surg 2014;67:119-23. [Crossref] [PubMed]
- Liu HL, Pang SY, Lee CC. Donor limb assessment after vascularized groin lymph node transfer for the treatment of breast cancer-related lymphedema: clinical and lymphoscintigraphy findings. J Plast Reconstr Aesthet Surg 2019;72:216-24. [Crossref] [PubMed]
- Viitanen TP, Maki MT, Seppanen MP, et al. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130:1246-53. [Crossref] [PubMed]
- Sulo E, Hartiala P, Viitanen T, et al. Risk of donor-site lymphatic vessel dysfunction after microvascular lymph node transfer. J Plast Reconstr Aesthet Surg 2015;68:551-8. [Crossref] [PubMed]


